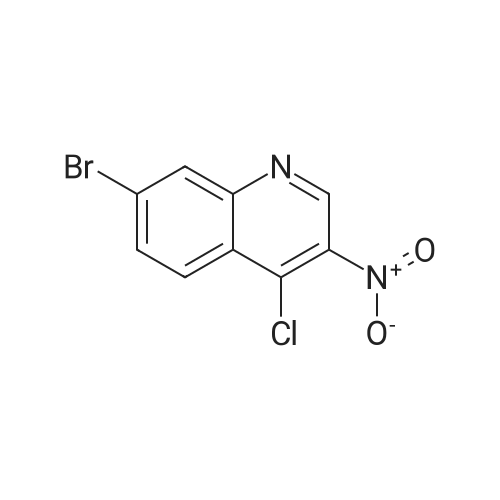
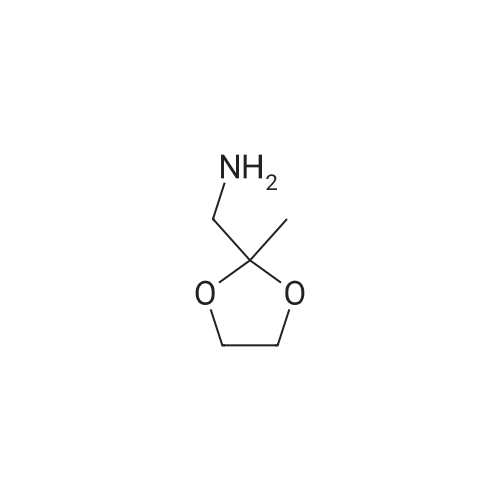
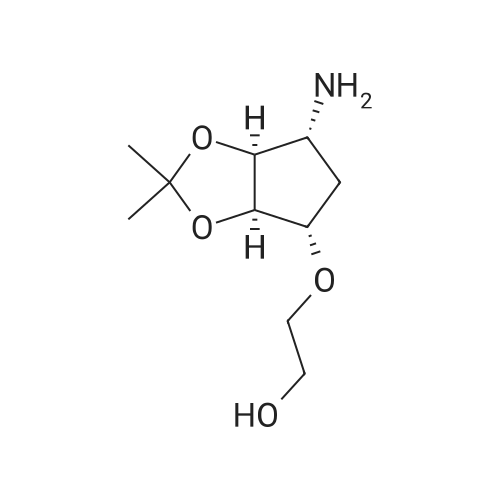
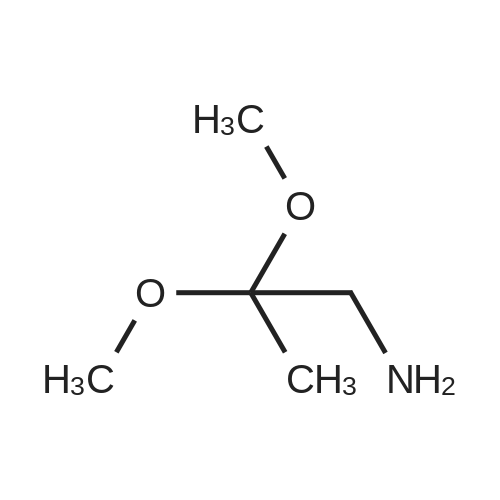
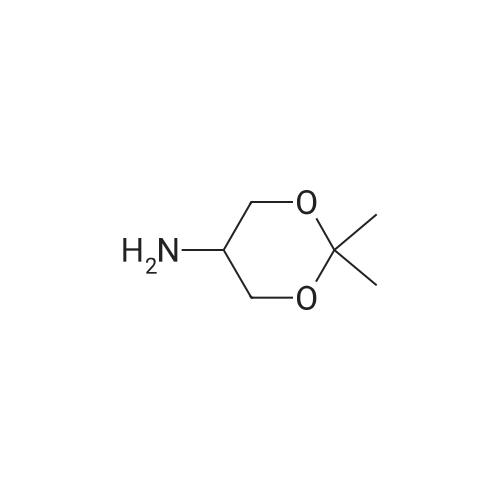
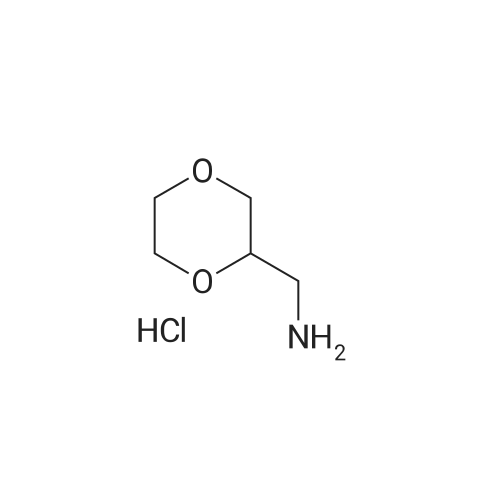
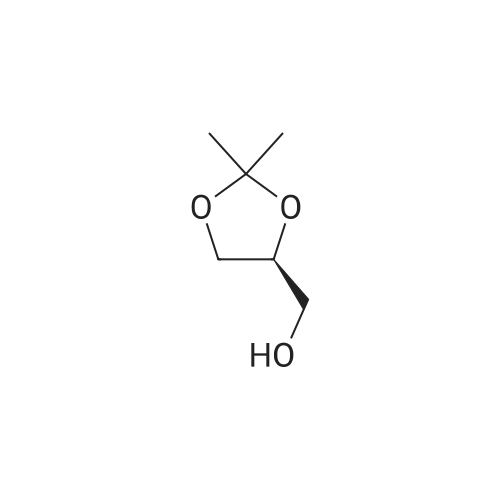
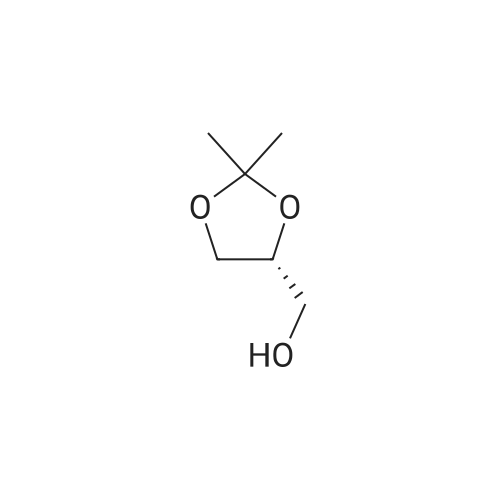
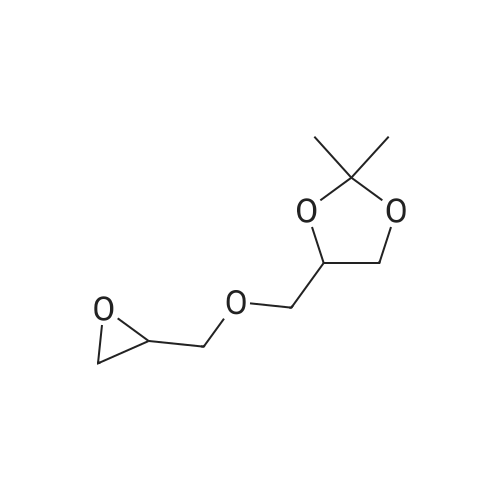
| 56% |
With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate; In N,N-dimethyl-formamide; at 20.0℃; for 16.0h; |
10300] In a typical run, (R)-2-amino-3-mercapto-3-meth- ylbutanoic acid (0.25 g, 1.68 mmol, 1 eq) and 1-(2,2- difluorobenzo[d] [1 ,3]dioxol-5-yl)cyclopropane-1 -carboxylic acid (0.45 g, 1.85 mmol, 1.1 eq) were taken up in DMF (10 mE) and HATU (0.83 g, 2.18 mmol, 1.3 eq) was added, followed by Et3N (0.508 g, 5.03 mmol, 3 eq). The resulting reaction mixture was stirred at room temperature for 16 hours. The following morning, the reaction mixture was extracted with EtOAc. The combined organic layers were washed with water, brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The resulting residue was purified by preparative HPEC afford (R)-2-(1 -(2,2- difluorobenzo[d] [1 ,3]dioxol-5-yl)cyclopropane-1 -carboxamido)-3-mercapto-3-methylbutanoic acid (0.368 g, 59percentyield) as a white solid. This material (0.368 g, 0.987 mmol, 1 eq) and (R)-(2,2-dimethyl-1 ,3-dioxolan-4-yl)methan- amine (0.155 g, 1.2 mmol, 1.1 eq) were taken up in DMF (5 mE) and HATU (0.487 g, 1.28 mmol, 1.3 eq) was added, followed by Et3N (0.299 g, 2.96 mmol, 3 eq). The resulting reaction mixture was stirred at room temperature for 16 hours. The following morning, the reaction mixture was extracted with EtOAc. The combined organic layers were washed with water, brine, dried over anhydrous Na2SO4 and concentrated and concentrated under reduced pressure. The resulting residue was purified by preparative HPEC to afford 1 -(2,2-difluorobenzo[d] [1 ,3]dioxol-5-yl)-N-((R)- 1 -((((R)2,2-dimethyl- 1 ,3-dioxolan-4-yl)methyl)amino)-3-mercapto-3-methyl-i -oxobutan-2-yl)cyclopropane-1 -carboxamide(0.27 g, 56percent yield) as a colorless oil. This material (0.27 g, 0.556 mmol, 1 eq) was taken up in DMF (2 mE) along with tert-butyl N-((4Z,7Z, 1 OZ, 1 3Z, 1 6Z, 1 9Z)-docosa-4,7, 10,13, 16,1 9-hexaenoyl)-S-(pyridin-2-ylthio)-E-cysteinate (0.365 g, 0.611 mmol, 1.1 eq) and methanol (2 mE) was added. tert-butyl N-((4Z,7Z, 1 OZ, 13Z, 1 6Z, 1 9Z)-Docosa-4,7, 10,13, 16,1 9-hexaenoyl)-S-(pyridin-2-ylthio)-E-cysteinate, in turn, was prepared according to the procedures outlined in example 3. The resulting reaction mixture was stirred at room temperature for 16 hours and then concentrated under reduced pressure. The resulting residue was purified by preparative HPEC to afford tert-butyl S?(((R)-3-(i-(2,2- difluorobenzo[d] [1 ,3]dioxol-5-yl)cyclopropane-i -carboxamido)-4-((((R)-2,2-dimethyl-i ,3-dioxolan-4-yl)methyl) amino)-2-methyl-4-oxobutan-2-yl)thio)-N-((4Z,7Z, 1 OZ, 1 3Z, 1 6Z, 1 9Z)-docosa-4,7, 10,13,16,1 9-hexaenoyl)-E-cysteinate (0.22 g, 40.8percent yield) as a colorless oil. This material (0.22 g, 0.467 mmol, 1 eq) was taken up in CH2C12 (5 mE) and TFA (1 mE) was added. The resulting reaction mixture was stirred at room temperature for 4 hour and then concentrated under reduced pressure. The resulting residue was purified by preparative HPEC to afford S?(()-3-(i -(2,2-difluorobenzo[d] [1 ,3]dioxol-5-yl)cyclopropane- 1-car- boxamido)-4-(((R)-2,3-dihydroxypropyl)amino)-2-methyl- 4-oxobutan-2-yl)thio)-N-((4Z,7Z, 1 OZ, 13Z, 1 6Z, 1 9Z)- docosa-4,7, 10,13,16,1 9-hexaenoyl)-E-cysteine (0.02 g, 10percent yield) as a colorless oil. MS, calculated for CH59F2N3O9S2: 875.37; found 876 [M+H]. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +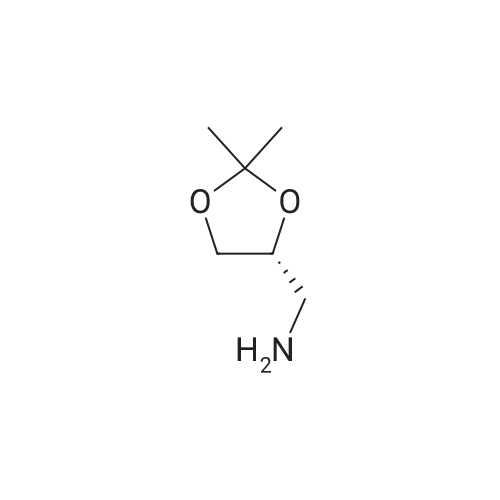

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping