|
With N-ethyl-N,N-diisopropylamine; In dimethyl sulfoxide; at 50.0℃; for 24.0h; |
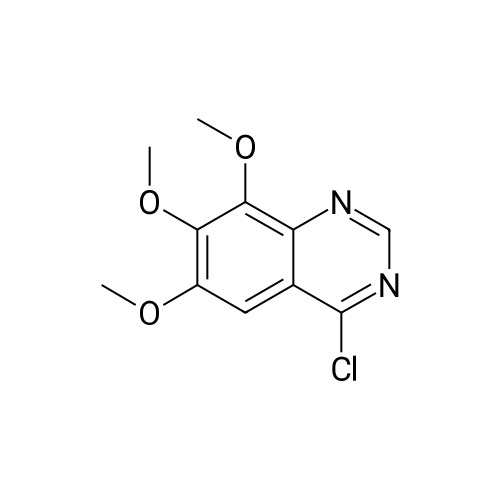
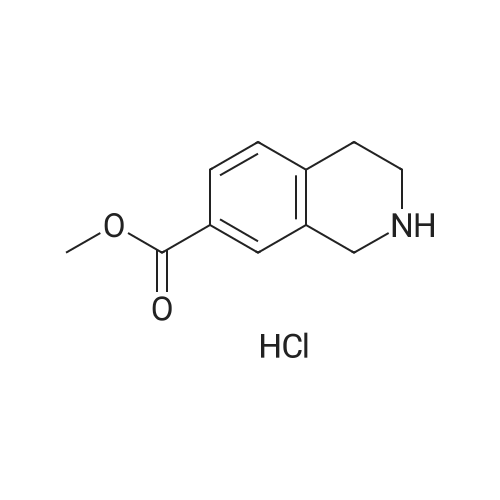
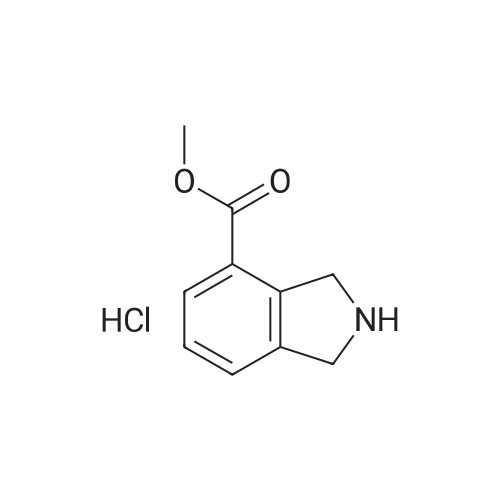
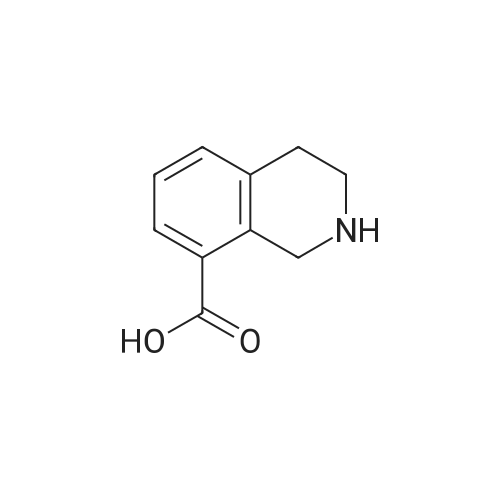
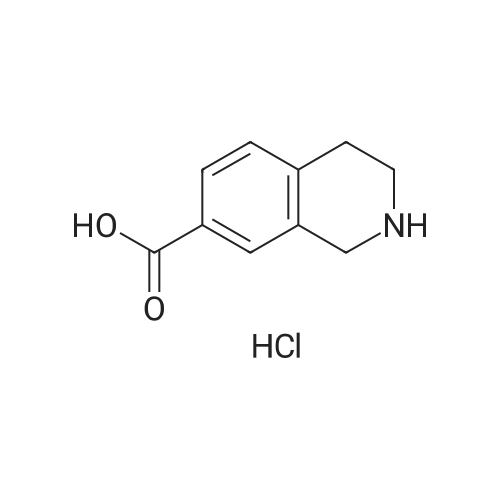
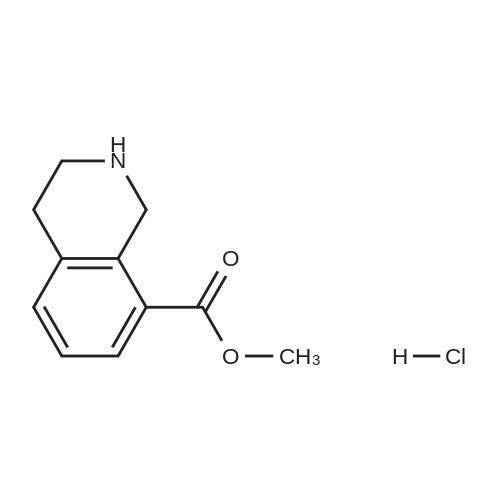
To a solution of methyl l,2,3,4-tetrahydroisoquinoline-8-carboxylate hydrochloride (12.37 g) and Example 1.4.4 (15 g) in dimethyl sulfoxide (100 mL) was added N,N-diisopropylethylamine(12 mL). The mixture was stirred at 50 C for 24 hours. The mixture was diluted with ethyl acetate (500 mL), washed with water and brine, and dried over Na2S04. After filtration and evaporation of the solvent, the crude material was purified via silica gel column chromatography, eluting with 20% ethyl acetate in hexane, to give the title compound. MS (ESI) m/e 448.4 (M+H)+. |
|
With N-ethyl-N,N-diisopropylamine; In dimethyl sulfoxide; at 50.0℃; for 24.0h; |
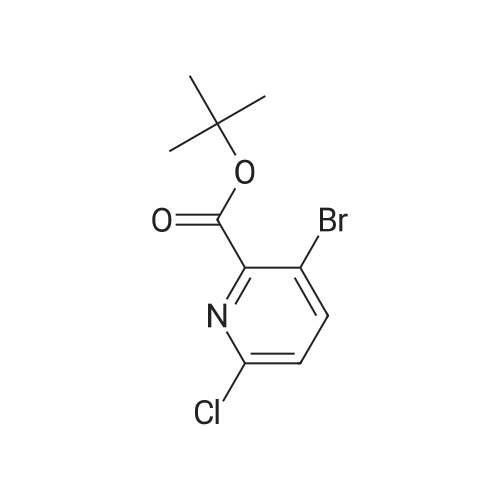
To a solution of <strong>[1029689-82-4]methyl 1,2,3,4-tetrahydroisoquinoline-8-carboxylate hydrochloride</strong> (12.37 g) and Example 1.1.10 (15 g) in dimethyl sulfoxide (100 mL) was added N,N-diisopropylethylamine (12 mL), and the mixture was stirred at 50 oC for 24 hours. The mixture was then diluted with ethyl acetate (500 mL) and washed with water and brine. The organic layer was dried over sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel chromatography, eluting with 20% ethyl acetate in hexane, to give the title compound. MS (ESI) m/e 448.4 (M+H)+. |
|
With N-ethyl-N,N-diisopropylamine; In dimethyl sulfoxide; at 50.0℃; for 24.0h; |
To a solution of <strong>[1029689-82-4]methyl 1,2,3,4-tetrahydroisoquinoline-8-carboxylate hydrochloride</strong> (12.37 g) and Example 1.1.10 (15 g) in dimethyl sulfoxide (100 mL) was added N,N-diisopropylethylamine (12 mL), and the mixture was stirred at 50 C. for 24 hours. The mixture was then diluted with ethyl acetate (500 mL) and washed with water and brine. The organic layer was dried over sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel chromatography, eluting with 20% ethyl acetate in hexane, to give the title compound. MS (ESI) m/e 448.4 (M+H)+. |
|
With N-ethyl-N,N-diisopropylamine; In dimethyl sulfoxide; at 50.0℃; for 24.0h; |
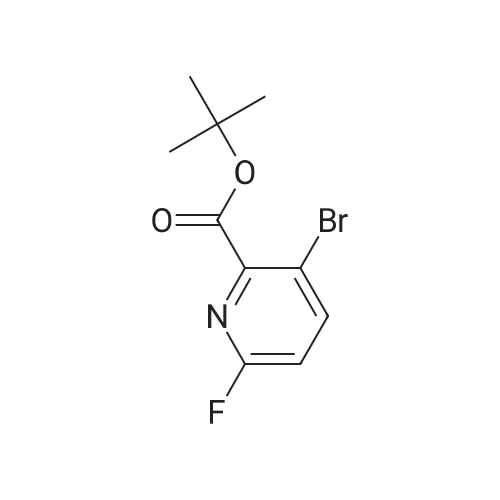
To a solution of <strong>[1029689-82-4]methyl 1,2,3,4-tetrahydroisoquinoline-8-carboxylate hydrochloride</strong> (12.37 g) and Example 1.1.11 (15 g) in dimethyl sulfoxide (100 mL) was added N,N-diisopropylethylamine (12 mL). The mixture was stirred at 50 C. for 24 hours. The mixture was then diluted with ethyl acetate (500 mL), washed with water and brine, and dried over Na2SO4. Filtration and evaporation of the solvent gave a residue that was purified by silica gel chromatography, eluting with 20% ethyl acetate in heptane, to give the title compound. MS (ESI) m/e 448.4 (M+H)+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping