| 36% |
With pyridine; In dichloromethane; at 20℃; for 12.0h; |
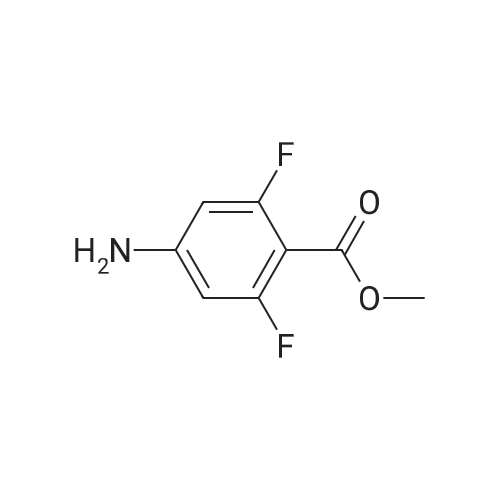
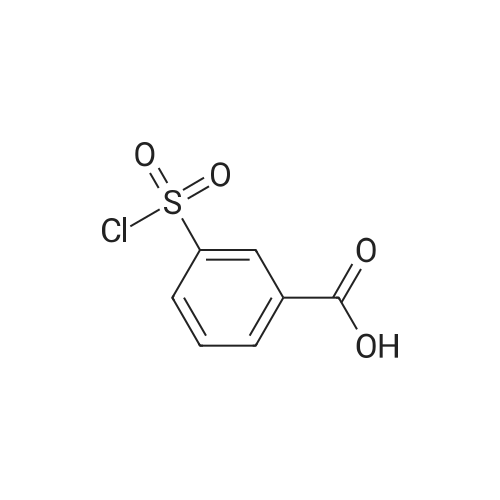
4-(Chlorosulfonyl)benzoic acid (25.0 g, 113 mmol) and <strong>[191478-99-6]methyl 4-amino-2,6-difluorobenzoate</strong> (19.0 g, 101 mmol) were dissolved in methylene chloride (500 ml), and pyridine (25.0 ml, 285 mmol) was added thereto, followed by stirring at room temperature for 12 hours. The reaction solution was concentrated under reduced pressure. The obtained residue was diluted with water, and then the pH was adjusted to 1.0 by adding 6 N hydrochloric acid. The precipitated solid was filtered, and washed with water. The obtained solid was resuspended in water, and washed with a saturated aqueous sodium hydrogen carbonate solution, followed by extraction with ethyl acetate (100 ml×2). The pH of the obtained aqueous layer was adjusted to 6.0 by adding 6 N hydrochloric acid thereto, followed by extraction with ethyl acetate (100 ml×2). The extraction liquids were combined, washed with saturated aqueous sodium chloride, and dried over anhydrous sodium sulfate. Then, the solvent was removed to obtain the title compound (15.0 g, 36%). [0213] 1H NMR (d-DMSO, 400 MHz): delta 11.50 (s, 1H), 8.14 (d, J=8.4 Hz, 2H), 8.01 (d, J=8.4 Hz, 2H), 6.67 (d, J=10.4 Hz, 2H), 3.87 (s, 3H); MS (ESI) m/z 372 (M+H)+ |
| 36% |
With pyridine; In dichloromethane; at 20℃; for 12.0h; |
(Step 1) 4-([3,5-Difluoro-4-(methoxycarbonyl)phenyl]amino}sulfonyl)benzoic acid (0430) (0431) 4-(Chlorosulfonyl)benzoic acid (25.0 g, 113 mmol) and <strong>[191478-99-6]methyl 4-amino-2,6-difluorobenzoate</strong> (19.0 g, 101 mmol) were dissolved in methylene chloride (500 ml), pyridine (25.0 ml, 285 mmol) was added thereto, and the resulting mixture was stirred at room temperature for 12 hours. The reaction solution was concentrated under reduced pressure, the obtained residue was diluted with water, a 6 N aqueous solution of hydrochloric acid was added for adjustment of the pH to 1.0, and the precipitated solid was filtered and washed with water. The obtained solid was suspended in water again, washed with a saturated aqueous solution of sodium hydrogen carbonate, and then extracted with ethyl acetate (100 ml×2). A 6 N aqueous solution of hydrochloric acid was added to the obtained aqueous layer for adjustment of the pH to 6.0, and ethyl acetate was added (100 ml×2) for extraction. The extracts were combined, washed with a saturated saline solution, dried over anhydrous sodium sulfate, and then the solvent was removed to obtain the title compound (15.0 g, 36%). (0432) 1H NMR (d-DMSO, 400 MHz): delta 11.50 (s, 1H), 8.14 (d, J=8.4 Hz, H), 8.01 (d, J=8.4 Hz, 2H), 6.67 (d, J=10.4 Hz, 2H), 3.87 (s, 3H); MS (ESI) m/z 372 (M+H)+ |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +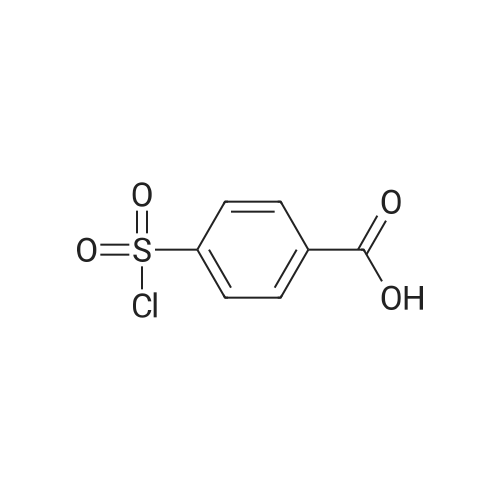

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping