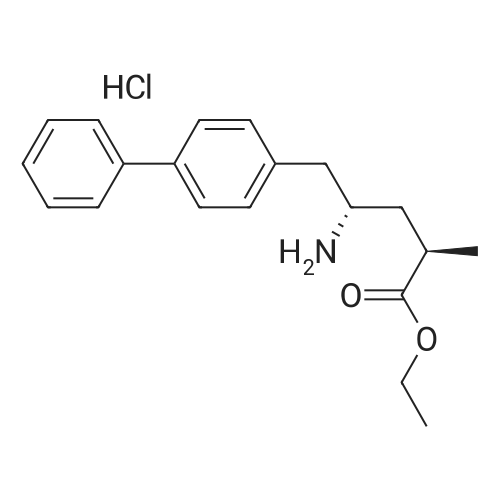
| 86.9% |
With hydrogen;diiodo(p-cymene)ruthenium(II) dimer; (SP,S?P)-1,1?-bis[bis(4-methoxy-3,5-dimethylphenyl)phosphino]-2,2?-bis[(R)-alpha-(dimethylamino)benzyl]ferrocene; In ethanol; at 40.0℃; under 15001.5 Torr; for 6.0h;Product distribution / selectivity; |
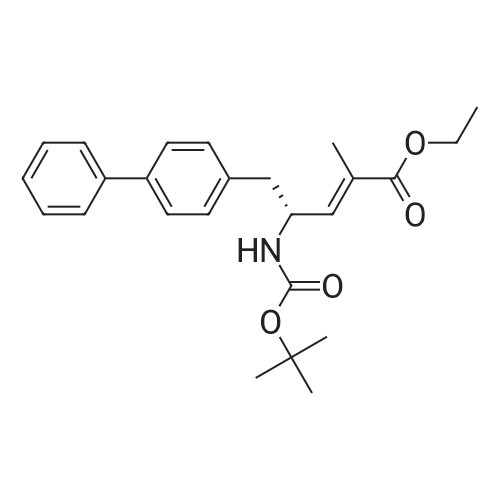
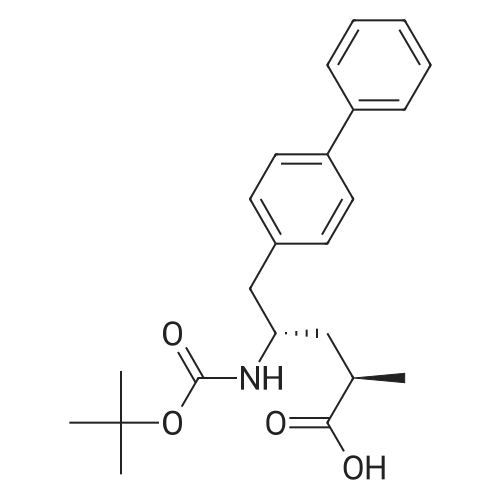
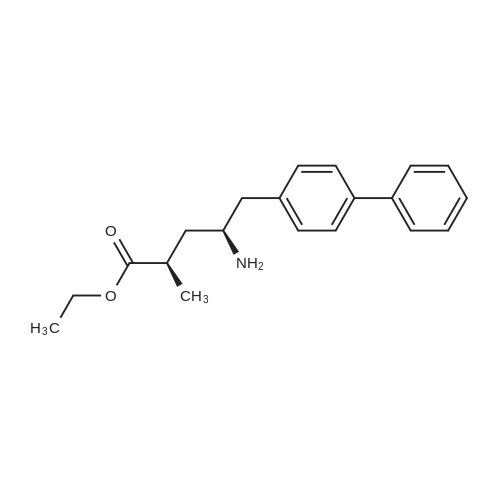
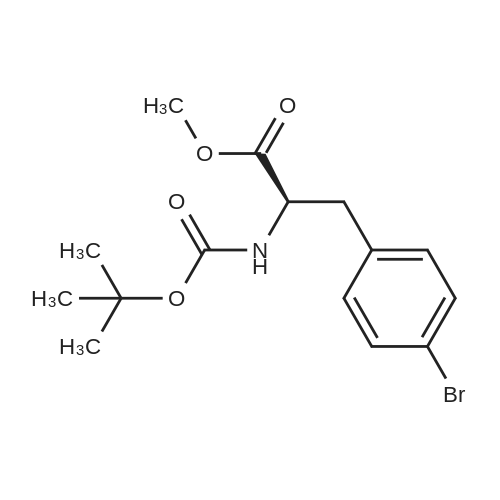
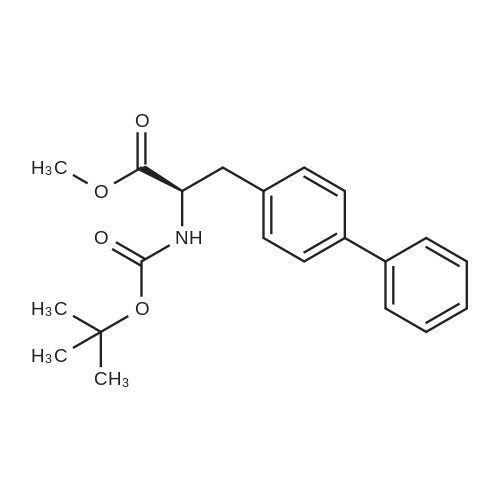
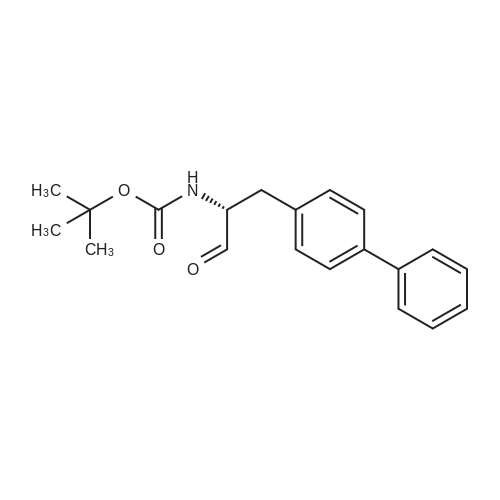
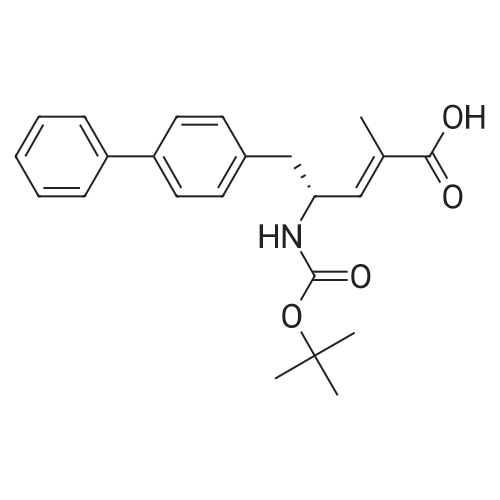
Example 2: (2R,4S)-5-biphenyl-4-yl-4-tert-butoxycarbonylamino-2-methylpentanoic acid in crystalline form; [Show Image] To a suspension of <strong>[1012341-48-8](E)-(R)-5-biphenyl-4-yl-4-tert-butoxycarbonylamino-2-methylpent-2-enoic acid</strong> (200 g, 524.3 mmol) in degassed ethanol (900 ml) at 40 C a solution of diiodo(p-cymene)ruthenium(II) dimer (0.052 g, 0.0524 mmol) and (alphaR,alphaR)-2,2'-bis(alpha-N,N-dimethylaminophenylmethyl)-(S,S)-1,1'-bis[di(3,5-dimethyl-4-methoxyphenyl)phosphine]ferrocene (= Mandyphos SL-M004-1) (0.116 g, 0.110 mmol) was added in degassed ethanol (100 ml). The solution was degassed using vacuum and a pressure of 20 bar hydrogen applied. The mixture was stirred at 40 C for 6 h. Vessel was then purged with nitrogen. Ethanol (700 ml) was removed by distillation. Isopropyl acetate (600 ml) was added. Solvent (600 ml) was removed by distillation. Isopropyl acetate (600 ml) was added. Solvent (600 ml) was removed by distillation. Isopropyl acetate (300 ml) was added and the solution heated to reflux. Heptane fraction (1200 ml) was added and the mixture cooled to room temperature. The solid was collected by filtration and washed with heptane fraction-isopropyl acetate 2 : 1 mixture (360 ml). The solid was dried overnight at 50 C under 1-50 mbar vacuum to afford (2R,4S)-5-biphenyl-4-yl-4-tert-butoxycarbonylamino-2-methylpentanoic acid (174.7 g, 86.9 %) as a white/off-white solid. Mpt 146-147 C; deltaH (500 MHz; DMSO) 1.07 (3H, d, J 7.0, 1-CH3), 1.34 (9H, s, (CH3)3), 1.38 (1H, m, 3-HA), 1.77 (1H, m, 3-HB), 2.43 (1H, m, 2-H), 2.70 (2H, d, J 7.0, 5-H), 3.69 (1H, m, 4-H), 6.74 (1H, d, J 9.0, NH), 7.27 (2H, d, J 8.0, Ar-ortho-H(Ph)), 7.36 (1H, t, J 7.0, Ar-(Ph)-para-H), 7.46 (2H, t, J 7.5, Ar-(Ph)-meta-H), 7.57 (2H, d, J 8.0, Ar-meta-H(Ph), 7.64 (2H, d, J 7.5, Ar-(Ph)-ortho-H), 12.01 (1H, s, CO2H); deltac (500 MHz, DMSO) 18.1 (1-CH3), 28.3 [(CH3)3], 35.9 (2-C), 37.9 (3-C), 40.7 (5-C), 50.0 (4-C), 77.4 [(C(CH3)3], 126.3, 126.5, 127.2, 128.9, 129.8 (Ar-CH), 137.7 (Ar-ipso-C(Ph)), 138.3 (Ar-para-C(Ph)), 140.1 (Ar-(Ph)-ipso-C), 155.2 (NCO), 177.2 (CO2H); mlz (+ESI) 406 ([MNa]+, 6%), 384 ([MH]+, 31), 328 (100), 284 (19); Found: [MH]+, 384.21691. C23H30NO4 requires MH 384.21693. Figure 1 shows the structure of crystalline (2R,4S)-5-biphenyl-4-yl-4-tert-butoxycarbonylamino-2-methylpentanoic acid measured by x-ray diffraction. The crystals comprise the following unit cell dimensions, measured by 100 K: |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping