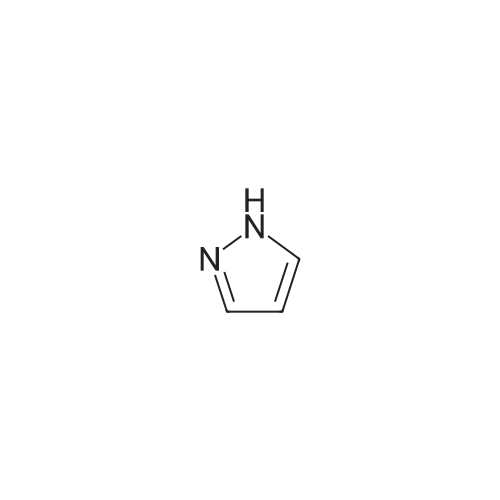
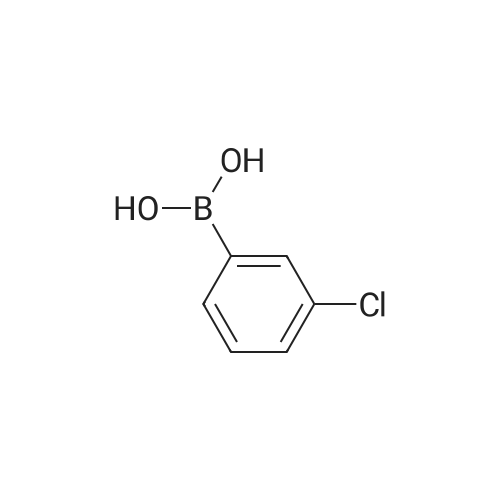
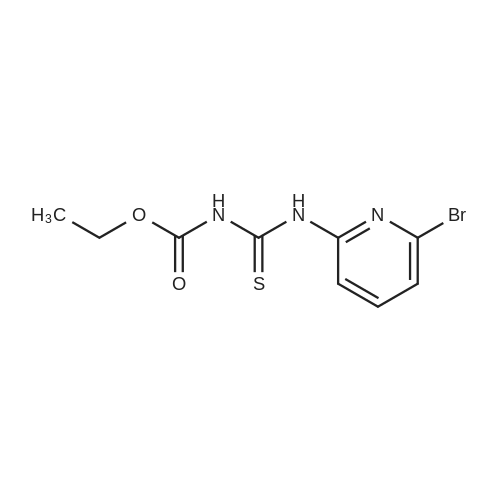
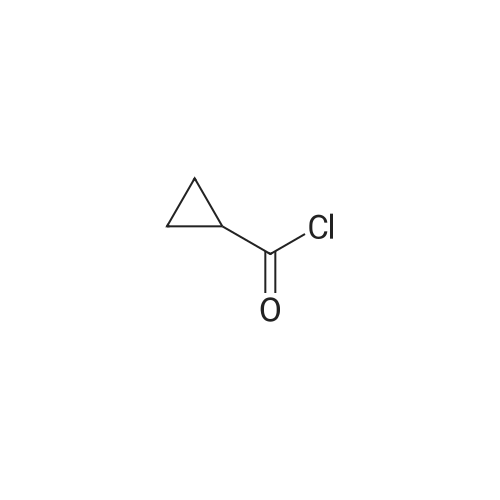
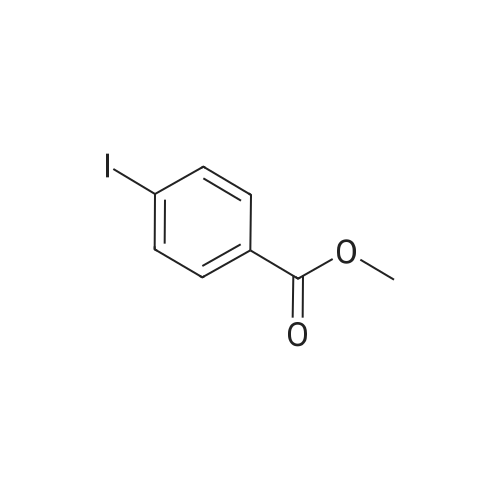
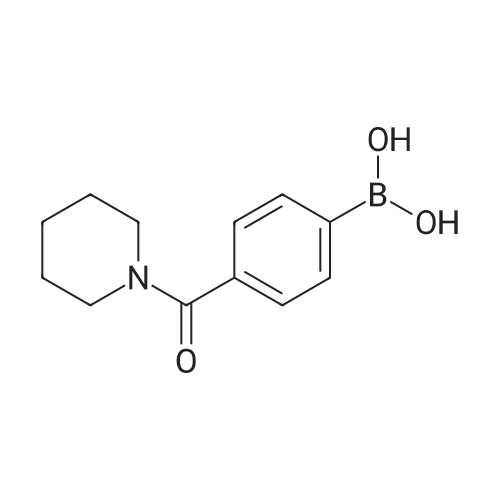
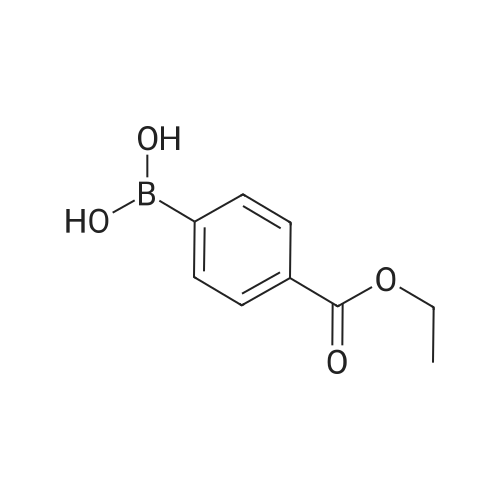
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In methanol; ethanol; at 20 - 60℃; for 5h; |
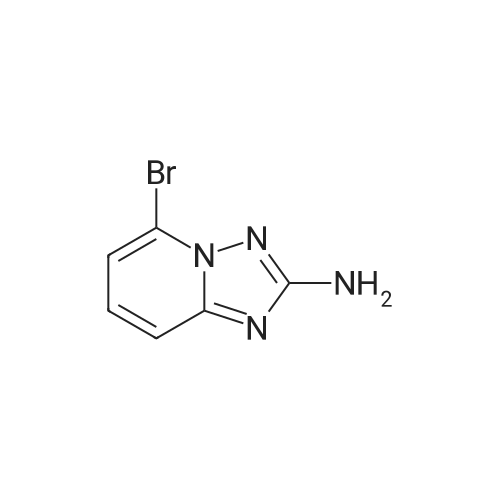
Compound 13To a stirred suspension of hydroxylamine hydrochloride (959.1 g, 13.8 mmol) and DIPEA (1.2 g, 9.2 mmol) in methanol/ethanol (1 : 1 , 50 ml) was added compound 12 (1.4 g, 4.6 mmol) as solid. The mixture was stirred at room temperature for 2 hours, followed by 3hours at 600C. The reaction mixture wasallowed to cool, diluted with CH2Cl2, which was then washed with water (2chi80 ml), the organic phase was evaporated and the residue was chromatographed in MeOH/ CH2Cl2 (8% -10%) to afford 750 mg of compound 13 as a white solid. LC-MS (ESI, positive): 215 [M+H]+. |
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In methanol; ethanol; at 20℃;Reflux; |
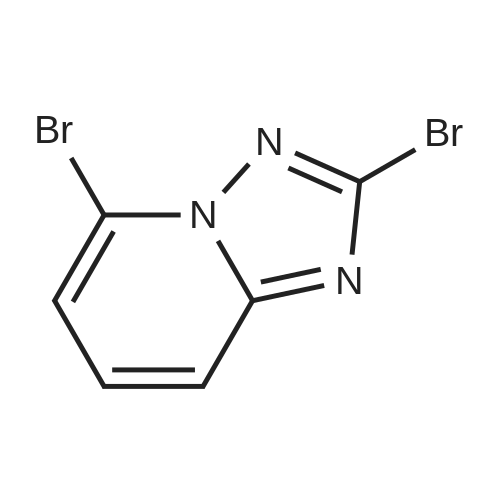
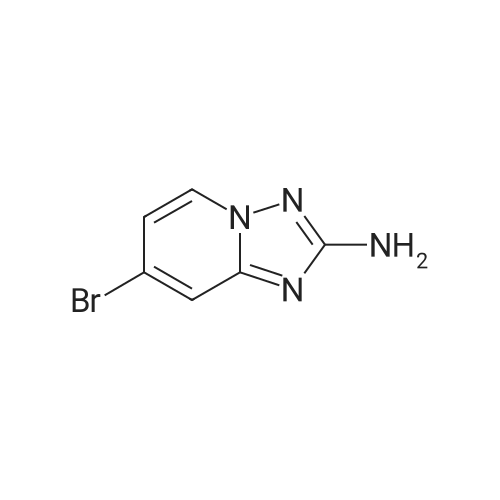
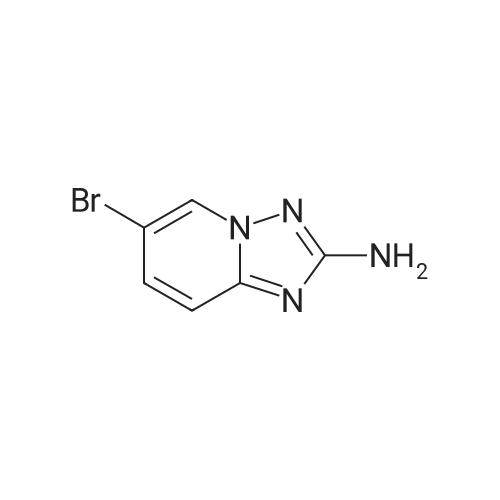
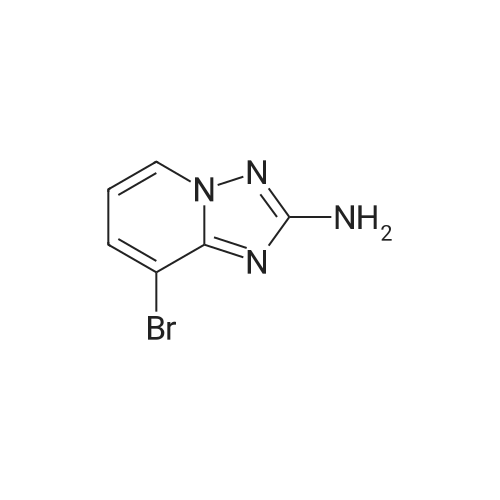
To a suspension of hydroxylamine hydrochloride (101.8 g, 1.465 mol) in EtOH/MeOH (1:1,900 mL) is added N,N-diisopropylethylamine (145.3 mL, 0.879 mol) and the mixture is stirred at room temp. (2O0C) for 1 h. l-(6-Bromo-pyridin-2-yl)-3-carboethoxy-thiourea (2) (89.0 g, 0.293 mol) may then be added and the mixture slowly heated to reflux (Note: bleach scrubber is required to quench H2S evolved). After 3 h at reflux, the mixture is allowed to cool and filtered to collect the precipitated solid. Further product may be collected by evaporation in vacuo of the filtrate, addition of H2O (250 mL) and filtration. The combined solids are washed successively with H2O (250 mL), EtOH/MeOH (1 :1, 250 mL) and Et2O (250 mL) then dried in vacuo to afford (3) as a solid. No further purification is required. 1H (400 MHz, DMSO-d6) delta: 7.43-7.34 (2H, m, 2 Hz aromatic-H), 7.24 (IH, dd, J 6.8 and 1.8 Hz, aromatic-H), 6.30 (2H, b, NH2); m/z 213/215 (1 :1, M+H+, 100%). |
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In methanol; ethanol; at 20℃;Reflux; |
To a suspension of hydroxylamine hydrochloride (101.8 g, 1.465 mol) in EtOH/MeOH (1:1, 900 mL) is added N,N-diisopropylethylamine (145.3 mL, 0.879 mol) and the mixture is stirred at room temp. (20 C.) for 1 h. 1-(6-Bromo-pyridin-2-yl)-3-carboethoxy-thiourea (2) (89.0 g, 0.293 mol) may then be added and the mixture slowly heated to reflux (Note: bleach scrubber is required to quench H2S evolved). After 3 h at reflux, the mixture is allowed to cool and filtered to collect the precipitated solid. Further product may be collected by evaporation in vacuo of the filtrate, addition of H2O (250 mL) and filtration. The combined solids are washed successively with H2O (250 mL), EtOH/MeOH (1:1, 250 mL) and Et2O (250 mL) then dried in vacuo to afford the triazolopyridine derivative (3) as a solid. The compound may be used as such for the next step without any purification. 1H (400 MHz, DMSO-d6) delta 7.43-7.34 (2H, m, 2* aromatic-H), 7.24 (1H, dd, J 6.8 and 1.8 Hz, aromatic-H), 6.30 (2H, br, NH2); m/z 213/215 (1:1, M+H+, 100%). |
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In methanol; ethanol; water; for 3h;Reflux; |
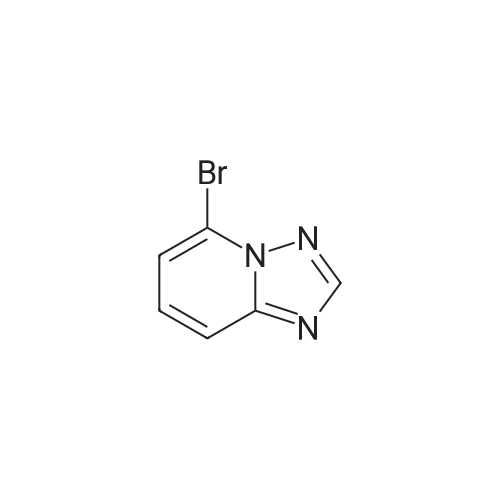
1.1.2 5-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine (3) To a suspension of hydroxylamine hydrochloride (101.8 g, 1.465 mol) in EtOH/MeOH (1:1, 900 mL) is added N,N-diisopropylethylamine (145.3 mL, 0.879 mol) and the mixture is stirred at room temp. (20 C.) for 1 h. 1-(6-Bromo-pyridin-2-yl)-3-carboethoxy-thiourea (2) (89.0 g, 0.293 mol) is then added and the mixture slowly heated to reflux (Note: bleach scrubber is required to quench H2S evolved). After 3 h at reflux, the mixture is allowed to cool and filtered to collect the precipitated solid. Further product is collected by evaporation in vacuo of the filtrate, addition of H2O (250 mL) and filtration. The combined solids are washed successively with H2O (250 mL), EtOH/MeOH (1:1, 250 mL) and Et2O (250 mL) then dried in vacuo to afford the triazolopyridine derivative (3) as a solid. The compound may be used as such for the next step without any purification. 1H (400 MHz, DMSO-d6) delta 7.43-7.34 (2H, m, 2*aromatic-H), 7.24 (1H, dd, J 6.8 and 1.8 Hz, aromatic-H), 6.30 (2H, br, NH2); m/z 213/215 (1:1, M+H+, 100%). |
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In methanol; ethanol; at 20℃; for 3h;Reflux; |
To a suspension of hydroxylamine hydrochloride (101.8 g, 1.465 mol) in EtOH/MeOH(1:1, 900 mL) was added N,N-diisopropylethylamine (145.3 mL, 0.879 mol) and the mixture wasstirred at room temp. (20 oq for 1 h. 1-(6-Bromo-pyridin-2-yl)-3-carboethoxy-thiourea (2) (89.0 g,0.293 mol) was then added and the mixture slowly heated to reflux (Note: bleach scrubber wasrequired to quench H2S evolved). After 3 h at reflux, the mixture was allowed to cool and filtered tocollect the precipitated solid. Further product was collected by evaporation in vacuo of the filtrate,addition of H20 (250 mL) and filtration. The combined solids were washed successively with H20 (250 mL), EtOH/MeOH (1:1, 250 mL) and Et20 (250 mL) then dried in vacuo to afford thetriazolopyridine derivative (3) as a solid. The compound was used as such in the next step without anypurification.[00159] 1H ( 400 MHz, DMSO-d6) 8 7.43-7.34 (2H, m, 2 x aromatic-H), 7.24 (lH, dd, J 6.8 and 1.8Hz, aromatic-H), 6.30 (2H, br, NH2); m/z 213/215 (1:1, M+H+, 100%). |
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In methanol; ethanol; for 3h;Reflux; |
1.1.2.2. Step ii): 5-Bromo-[1,2,4]triazolo[1,5-a]kyridin-2-ylamine To a suspension of hydroxylamine hydrochloride (101.8 g, 1.465 mol) in EtOH/MeOH (1:1, 900 mL) is added N,N-diisopropylethylamine (145.3 mL, 0.879 mol) and the mixture is stirred at room temp. (20 C.) for 1 h. 1-(6-Bromo-pyridin-2-yl)-3-carboethoxy-thiourea (2) (89.0 g, 0.293 mol) is then added and the mixture slowly heated to reflux (Note: bleach scrubber is required to quench H2S evolved). After 3h at reflux, the mixture is allowed to cool and filtered to collect the precipitated solid. Further product is collected by evaporation in vacuo of the filtrate, addition of H2O (250 mL) and filtration. The combined solids are washed successively with H2O (250 mL), EtOH/MeOH (1:1, 250 mL) and Et2O (250 mL) then dried in vacuo to afford the triazolopyridine derivative (3) as a solid. The compound may be used as such for the next step without any purification. 1H (400 MHz, DMSO-d6) delta 7.43-7.34 (2H, m, 2×aromatic-H), 7.24 (1H, dd, J6.8 and 1.8 Hz, aromatic-H), 6.30 (2H, br, NH2); m/z 213/215 (1:1, M+H+, 100%). |
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In methanol; ethanol; at 20℃;Reflux; |
Step ii : 5-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine To a suspension of hydroxylamine hydrochloride (101.8 g, 1.465 mol) in EtOH/MeOH (1:1, 900 mL) is added N,N-diisopropylethylamine (145.3 mL, 0.879 mol) and the mixture is stirred at room temp. (20 C.) for 1 h. 1-(6-Bromo-pyridin-2-yl)-3-carboethoxy-thiourea (2) (89.0 g, 0.293 mol) is then added and the mixture slowly heated to reflux (Note: bleach scrubber is required to quench H2S evolved). After 3 h at reflux, the mixture is allowed to cool and filtered to collect the precipitated solid. Further product is collected by evaporation in vacuo of the filtrate, addition of H2O (250 mL) and filtration. The combined solids are washed successively with H2O (250 mL), EtOH/MeOH (1:1, 250 mL) and Et2O (250 mL) then dried in vacuo to afford the triazolopyridine derivative (3) as a solid. The compound may be used as such for the next step without any purification. 1H (400 MHz, DMSO-d6) delta 7.43-7.34 (2H, m, 2* aromatic-H), 7.24 (1H, dd, J 6.8 and 1.8 Hz, aromatic-H), 6.30 (2H, br, NH2); m/z 213/215 (1:1, M+H+, 100%). |
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In methanol; ethanol; for 3h;Reflux; |
[0165] To a suspension of hydroxylamine hydrochloride (101.8 g, 1.465 mol) in EtOH/MeOH (1 : 1, 900 mL) is added N,N-diisopropylethylamine (145.3 mL, 0.879 mol) and the mixture is stirred at room temp. (20 C) for 1 h. l-(6-Bromo-pyridin-2-yl)-3-carboethoxy-thiourea (2) (89.0 g, 0.293 mol) is then added and the mixture slowly heated to reflux (Note: bleach scrubber is required to quench H2S evolved). After 3 h at reflux, the mixture is allowed to cool and filtered to collect the precipitated solid. Further product is collected by evaporation in vacuo of the filtrate, addition of H20 (250 mL) and filtration. The combined solids are washed successively with H20 (250 mL), EtOH/MeOH (1 : 1, 250 mL) and Et20 (250 mL) then dried in vacuo to afford the triazolopyridine derivative (3) as a solid. The compound may be used as such for the next step without any purification. [0166] lH (400 MHz, DMSO-dg) delta 7.43-7.34 (2H, m, 2 x aromatic-H), 7.24 (1H, dd, J 6.8 and 1.8 Hz, aromatic-H), 6.30 (2H, br, NH2); m/z 213/215 (1 : 1, M+H+, 100%). |
| 6 g |
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In ethanol; at 20℃; for 4h;Reflux; |
Step 2, 5-bromo-[1,2,4]triazolo[1,5a]pyridin-2-ylamine 10.0g of hydroxylamine hydrochloride was dissolved in 100 ml of ethanol, 14.5 ml of N,N-diisopropylethylamine was added, and the reaction was stirred at room temperature for 1 hour. 9 g of the product of step 1 was then added, and the mixture was heated under reflux. After 3 hours, the mixture was cooled and solid was precipitated. The solid was filtered, washed, and air-dried to afford 6 g of the desired product. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping