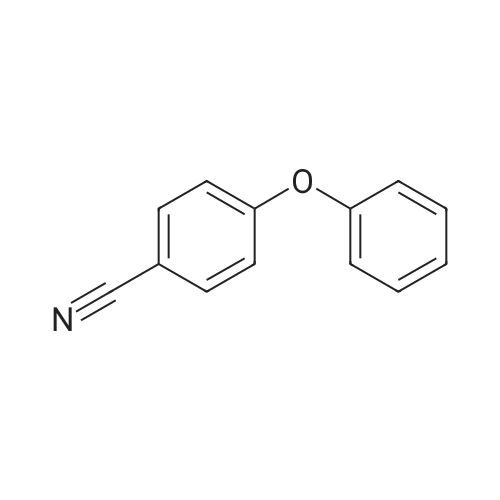
|
With bromine;aluminum (III) chloride; at 25 - 59℃; for 2.08333 - 25.0833h;Product distribution / selectivity; |
Comparative Example; A 500 milliliter four-neck round bottom flask was fitted with a mechanical stirrer, a double walled reflux condenser, a thermocouple, a temperature controller, a heating mantle, and a syringe pump fitted with a Teflon needle. The flask was vented to a water trap for collection of by-product hydrogen bromide. Dry bromine (929.5 grams, 5.82 moles, 200% excess) was charged into the reaction flask, followed by 4.1 grams of aluminum chloride (0.031 mole). The reaction was stirred for 5 minutes.Addition of 33.0 grams (0.19 mole) of diphenyl ether was initiated to the bromine-catalyst mixture at a temperature of 25C. The diphenyl ether addition was maintained at a constant rate by use of a syringe pump over a period of about 180 minutes. The reaction temperature was allowed to increase by way of exotherm to about 35C. Additional heat was applied after the diphenyl ether addition had been completed, and the reaction temperature increased to about 59C within about 20 minutes. After 180 minutes of post addition heating, the heat input was removed and the reaction allowed to cool to room temperature in about 90 minutes.A two liter four-neck round bottom flask was fitted with a mechanical stirrer, a distilling head, a double walled reflux condenser, a thermocouple, a temperature controller, and a heating mantle. One liter of water and the reaction slurry were charged to the flask and the excess bromine was distilled off until a temperature of 100C was achieved.Decabromodiphenyl ether was filtered from the aqueous slurry, washed with water, and dried at 100 C. in a forced air oven.Gas chromatographic analysis of the resulting product showed decabromodiphenyl ether 96.93 area percent, nonabromodiphenyl ether isomers totaling 2.79%, octabromodiphenyl ether isomers totaling 0.25%, and heptabromodiphenyl ether isomers totaling 0.02%.; Example 1; A two liter four-neck round bottom flask was fitted with a mechanical stirrer, a double walled reflux condenser, a thermocouple, a temperature controller, a heating mantle, and a syringe pump fitted with a Teflon needle. The flask was vented to a water trap for collection of by-product hydrogen bromide. Dry bromine (3,410 grams, 21.34 moles, 1000% excess) was charged into the reaction flask, followed by 17.9 grams of aluminum chloride (0.13 mole). The reaction was stirred for five minutes.Addition of 33.0 grams (0.19 mole) of diphenyl ether was initiated to the bromine-catalyst mixture at a temperature of 25 C. The diphenyl ether addition was maintained at a constant rate by use of a syringe pump over a period of about 60 minutes. The reaction temperature was allowed to increase by way of exotherm to about 35 C. Additional heat was applied after the diphenyl ether addition had been completed, and the reaction temperature increased to about 59 C. within about 20 minutes. After about 60 minutes of post addition heating, the heat input was removed and the reaction allowed to cool to room temperature in about 90 minutes.A three liter four-neck round bottom flask was fitted with a mechanical stirrer, a distilling head, a double walled reflux condenser, a thermocouple, a temperature controller, and a heating mantle. One liter of water and the reaction slurry were charged to the flask and the excess bromine was distilled off until a temperature of 100 C. was achieved.Decabromodiphenyl ether was filtered from the aqueous slurry, washed with water, and dried at 100 C. in a forced air oven.Gas chromatographic analysis of the resulting product showed decabromodiphenylether 99.95 area percent, nonabromodiphenyl ether isomers totaling 0.05%, with no other isomers present.; Example 2; The procedure of Example 1 was repeated except that the amount of aluminum chloride was reduced to 6.2 grams (0.047 mole).Gas chromatographic analysis of the resulting product showed decabromodiphenylether 99.90 area percent and nonabromodiphenyl ether 0.1%, with no other isomers present.; Example 3; A two liter four-neck round bottom flask was fitted with a mechanical stirrer, a double-walled reflux condenser, a thermocouple, a temperature controller, a heating mantle, and a syringe pump fitted with a Teflon needle. The flask was vented to a water trap for collection of by-product hydrogen bromide. Dry bromine (3410.1 grams, 21.34 moles, 1000% excess) was charged into the reaction flask, followed by 6.5 grams of aluminum chloride (0.049 mole). The reaction was stirred for five minutes.Addition of 33.0 grams (0.19 mole) of diphenyl ether was initiated to the bromine-catalyst mixture at a temperature of 25 C. The diphenyl ether addition was maintained at a constant rate by use of a syringe pump over a period of about 60 minutes. The reaction temperature was allowed to increase by way of exotherm to about 31 C. Additional heat was applied after the diphenyl ether addition had been completed, and the reaction temperature increased to about 59 C. within about 20 minutes. After about 24 hours of post... |
| 99.436 - 99.71%Chromat. |
|
EXAMPLE 6; Preparation of Partially brominated DPO[0056] To the 500 mL flask (equipped as described in Example 4) containing 47.3 g of DPO was added over about 10 minutes, 28.0 g of bromine with stirring and cooling at room temperature. Catalyzed Bromination [0057] In a 1 -liter jacketed flask equipped with mechanical stirrer, Friedrich condenser(water cooled at about 250C), and with a 1/32-inch diptube but without a fractionation column were placed 3.8 g of AlCl3 and 885 g of bromine. After a feed time of about 7.25 hours, all of the partially brominated DPO had been fed from the flask. The reaction temperature was maintained at 56.30C to 57.20C throughout the addition. The reaction mixture was refluxed for 4 minutes as the temperature rose to 58.40C, then 450 mL of water was added and the reactor was set for distillation. The product was distilled to 1700F (about 770C) and 312 g of bromine was collected. The water layer was decanted from the reactor, 400 mL of water was added, stirred, and discarded. Then 400 mL of water and 10 g of NaOH were added, the mixture was stirred well and product was collected and water washed on a filter. GC analysis showed the product was composed of 99.71% of decabromodiphenyl oxide, and 0.034 and0.259% of the first and second nonabromodiphenyl oxide isomers, respectively. The product was placed in a 1250C oven and after drying overnight weighed 252.0 g. EXAMPLE 7Preparation of Partially brominated DPO [0058] To the 500 mL flask (equipped as described in Example 4) containing 49.1 g of DPO <n="14"/>was added over aboutlO minutes, 29.7 g of bromine. This was purged with nitrogen to remove HBr. Catalyzed Bromination[0059] In a 1 -liter jacketed flask equipped as in Example 6 (no fractionation column) were placed 3.8 g of AlCl3 and 884 g of bromine. The mixture was heated to 59C and a feed of the partially brominated DPO formed above was initiated. The feed through the 1/32-inch diptube was set at a rate of about 0.21 mL per minute. All the partially brominated DPO was added over 3 hours and 23 minutes, The reaction mixture had been maintained at a temperature of 56.10C to 57.10C throughout the addition time reflux was continued for about 10 more minutes as the temperature rose to 59.60C. Then 450 mL of water was added to the reaction mixture and the reactor was set for distillation. The mixture was distilled to 770C and 294.5 g of bromine was collected. The mixture was worked up as in Example 6. GC analysis of the product showed 99.59% of decabromodiphenyl oxide and 0.11% and 0.296% of first and second nonabromodiphenyl oxide peaks, respectively. Present in the product were a few "lumps". One was removed and a GC showed it contained 99.61% of decabromodiphenyl oxide and 0.100 and 0.291% of the first and second nonabromodiphenyl oxide isomers, respectively. The product was oven dried. EXAMPLE 8Preparation of Partially brominated DPO [0060] To the 500 mL flask (equipped as in Example 4) containing 49.00 g of DPO was added 31.4 g of bromine over about 10 minutes. Then the mixture was purged with nitrogen. Catalyzed Bromination[0061] In a 1 -liter jacketed flask, equipped as in Example 6, were placed 3.82 g OfAlCl3 and 988 g of bromine The mixture was heated to 56.O0C and addition of the partially brominated DPO begun at a feed rate of about 0.18 mL per minute. This feed was maintained for a period of about 4 hours with the temperature fluctuating between 53.O0C and 54.O0C. The mixture was allowed to reflux for about another 7 minutes with the temperature reaching about 600C. Then, 450 mL of water was added to the reaction mixture and the reaction vessel was set for distilling bromine. The distillation was conducted to 770C whereby an amount of 400.2 g of bromine was recovered. Product was isolated as in Example 5 and oven dried. GC analysis showed 0.093% and 0.471% of the first and second nonabromodiphenyl oxide peaks, respectively, and 99.436% of decabromodiphenyl oxide. After drying over the weekend the product weighed 260.1 g. |
|
With bromine;aluminum (III) chloride; In dichloromethane; chlorobromomethane; 1,2-dibromomethane; at 7℃; for 3.5 - 8h;Heating / reflux;Product distribution / selectivity; |
Example 1; Decabromodiphenyl ether - Simultaneous bromination and milling in a mixed solvent; To a 1 liter round bottomed flask equipped with a mechanical stirrer, a dropping funnel, a thermocouple and a reflux condenser was added 440 g of solvent mixture (DCM 20 %, CBM 40 %, and DBM 40 % w/w) ; bromine, 475 g; AlCl3,4.3 g; and ceramic beads (1.5-3.5 mm diameter), 814 g-A solution of diphenyl oxide (42.5 g, ) in 20 ml of solvent mixture was dropped into the flask during 70 minutes with stirring while keeping the temperature at 7- 13 C . The reaction mixture was refluxed for 4.5 hours, the flask was cooled and 55 ml of water was carefully added to destroy the catalyst. Excess bromine was bleached with sodium bisulfite solution, the aqueous phase was separated and the organic phase was washed with water. The product mixture was passed through a sieve to remove the ceramic beads and the mixture was filtered, washed with water and dried. The product comprised 99.4 % decabromodiphenyl ether and 0.1 % nonabromodiphenyl ether (in the Examples sometimes abbreviated "Deca" and "Nona", respectively). Particle size 7.1 microns (dg0) .; Example 2 (comparative); Decabromodiphenyl ether - Bromination without milling in a mixed solvent.; The procedure of Experiment 1 was repeated without the ceramic beads present. The product consisted of 94.1 % Deca and 5.8 % Nona. Particle size 98 microns (d90).; Example 3; Decabromodiphenyl ether- increasing the crystal size of milled Deca in a simulated reaction mixture after bromination and destruction of the catalyst.; The product of Example 1, having a particle size of 7.1 microns (dgo) and an assay of 99.4 %, was charged to a 1 liter flask followed by 440 g of solvent mixture (DCM 20 %, CBM 40 %, and DBM 40 %); 4.3 g AlCl3, 50 g of bromine and 50 ml of water to destroy the catalyst. The mixture was refluxed for 5.3 hours, cooled and bleached with bisulfite. The product was filtered, washed with water and dried. The particle size v/as 28.8 microns (dgo), had an assay of 99.7 % and was easily filtered.; Example 7; Decabromodiphenyl ether - Bromination of pre-milled Deca precursor; To a 2 liter jacketed reaction vessel equipped with a mechanical stirrer, a dropping funnel, a thermocouple and a reflux condenser was added 500 g of milled Deca (content 97.3 %, particle size 3.8 microns (d90) ) , 1945 g of solvent mixture, 444 g bromine and 22 g AICI3. The <n="20"/>mixture was heated at reflux for 3.5 hours. Water, 260 ml, was added and the excess bromine was bleached with sodium bisulfite solution. To the thick slurry was added 150 ml of water. The mixture was filtered and the dried solid comprised 99.6 % Deca with particle size 38.5 microns (dgo).; Example 8; Decabromodiphenyl ether- Bromination of pre-milled Deca precursor on an industrial scale; A reactor vessel of 16 cubic meters capacity was charged with 7500 liters of solvent consisting of 12.6 % dichloromethane, 32.5 % bromochloromethane and 54.9 % dibromomethane . Aluminum chloride, 150 kg, 8 tons of pre- milled Deca (Deca content 97.9 % with particle size 14 microns (dg0) ) and 1000 kg of bromine were added. The mixture was heated at reflux for 8 hours and was then bleached with 1340 liters of 38 % sodium bisulfite solution. The upper aqueous solution was decanted and the mixture was washed with two 1200 liters portions of water. Sodium hydroxide, 20 %, was added to neutralize the mixture which was then centrifuged. The product was dried and was found to contain 99.3 % Deca with particle size 42 microns (d90).; Example 9; Decabromodiphenyl ethane - Simultaneous bromination and milling in a mixed solvent; To a 1 liter round bottomed flask equipped with a mechanical stirrer, a dropping funnel, a thermocouple and a reflux condenser was added 520 g of solvent mixture <n="21"/>(DCM 6 %, CBM 20 %, and DBM 74 % ) ; bromine, 539 g; AlCl3, 9 g; and ceramic beads (1.5-3.5 mm diameter), 840 g.A 55 % solution of diphenyl ethane in DCM (91.1 g, ) was dropped into the flask during 30 minutes with stirring while keeping the temperature at 21-26 0C. The reaction mixture was refluxed for 6.7 hours, and 120 ml of water was carefully added to destroy the catalyst. Bromine was bleached with sodium bisulfite solution, the aqueous phase was separated and the organic phase was washed with water and was neutralized with 20 % NaOH. The product mixture was passed through a sieve to remove the ceramic beads and the mixture was filtered, washed with water and dried. The product comprised 91.4 % decabromodiphenyl ethane and 7.8 % nonabromodiphenyl ethane. Particle size was 6.1 microns (d90).; Example 10 (comparative); Decabromodiphenyl ethane - Bromination without milling in a mixed solvent.; The procedure of Example 9 was repeated without the ceramic beads present. The product comprised 80.6 % decabromodiphenyl ethane and 18.6 % nonabromodiphenyl ethane. Particle size 22 .microns (dgo). |
|
With bromine;aluminum (III) chloride; at 6℃; for 7.2h;Heating / reflux;Product distribution / selectivity; |
Example 4; Decabromodiphenyl ether- Simultaneous bromination and milling in bromine solvent; To a 1 liter round bottomed flask equipped with a mechanical stirrer, a dropping funnel, a thermocouple and a reflux condenser was added 1200 g bromine, 6.8 g AlCl3, and 840 g ceramic beads (1.5-3.5 mm diameter). Molten DPO, 60 g was dropped into the flask from the heated funnel during 1 hour while keeping the temperature at about 6 0C. The contents of the flask were heated at reflux for' 6.2 hours and were then cooled to room temperature. Water, 100 ml, was carefully added to destroy the catalyst. The excess bromine was then distilled with the simultaneous addition of 165 ml of water. Some residual bromine was bleached with sodium bisulfite solution and the product mixture was passed <n="19"/>through a sieve to remove the ceramic beads. The mixture was filtered, washed with water and dried. The product comprised 99.9 % Deca and 0.1 % Nona. Particle size 14 microns (dgo).; Example 5 (comparative); Decabromodiphenyl ether- Bromination without milling in bromine solvent.; The procedure of Experiment 4 was repeated without the ceramic beads present. The product comprised 98.3 % Deca and 1.2 % Nona. Particle size 143 microns (d90). |
|
With bromine;aluminum tri-bromide; for 5h;Heating / reflux;Product distribution / selectivity; |
Example 12 (comparative)Decabromodiphenyl ether - Bromination of Deca completely dissolved in bromine.; The solubility of Deca in bromine was determined as 2.64 g Deca in 100 g bromine at 200C.To a 1 liter round bottomed flask equipped with a mechanical stirrer, a dropping funnel, a thermocouple and a reflux condenser was added 22 g of non milled Deca (content 97.1 %) and 1642 g bromine to produce a 1.32 % solution. AlBr3, 14.1 g, was added and the mixture was heated at reflux for 5 hours. After cooling to room temperature, water, 250 ml, was carefully added. The bromine was distilled and an additional 520 g of water was added. The solid was filtered and dried and consisted of 99.6 % Deca. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping