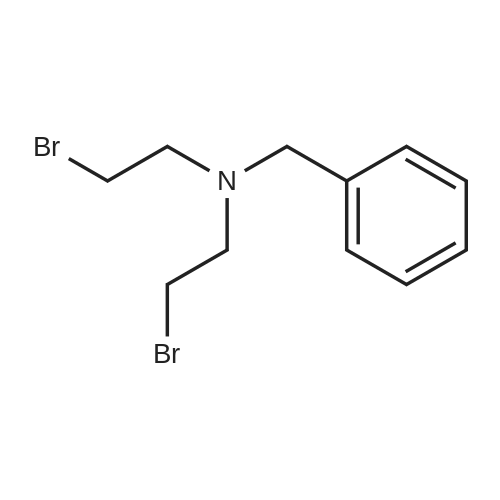
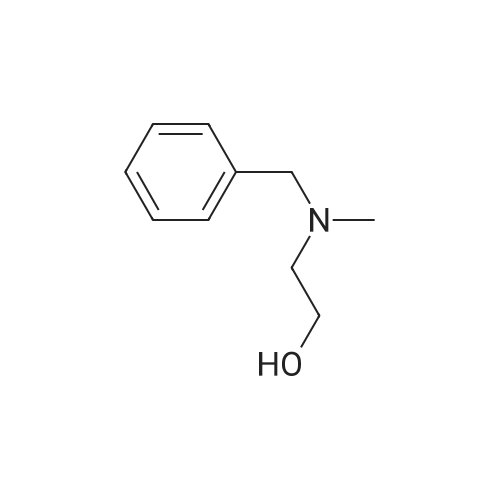
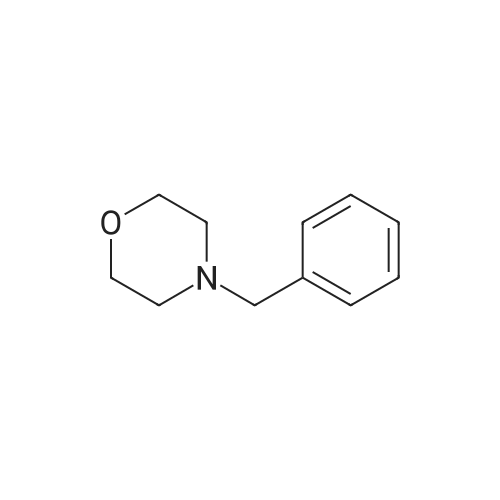
| 54.6% |
With phosphorus tribromide; In toluene; at 0℃; for 6h;Inert atmosphere; Reflux; |
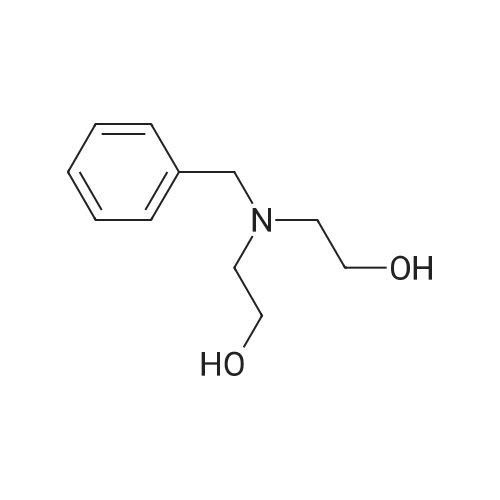
A mixture of compound O-1 (12.10 g, 115.1 mmol), benzyl bromide (13.70 mL, 115.3 mmol) and potassium carbonate (31.81 g, 230.2 mmol) in acetone (120 mL) was refluxed for 18 hours, cooled to rt and filtered. The filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (DCM/MeOH (v/v) = 20/1) to give compound O-2 as pale yellow liquid (16.39 g, 72.9%). The compound was characterized by the following spectroscopic data: 1H NMR (400 MHz, CDCl3): delta 7.36-7.23 (m, 5H), 3.70 (s, 2H), 3.62 (t, J = 5.3 Hz, 4H), 2.72 (t, J = 5.3 Hz, 4H), 2.48 (brs, 2H). [0555] To a solution of compound O-2 (14.60 g, 74.77 mmol) in toluene (140 mL) was added phosphorus tribromide (21.1 mL, 224.5 mmol) dropwise at 0 C under N2. At the end of the addition, the mixture was refluxed for 6 hours, cooled to rt, quenched with ice water (400 mL) and filtered. The filtrate was washed with NaOH solution and extracted with DCM (100 mL x 3). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane/EtOAc (v/v) = 10/1) to give the title compound 32-1 as colorless oil (13.30 g, 54.6%). The compound was characterized by the following spectroscopic data: 1H NMR (400 MHz, CDCl3): delta 7.36-7.25 (m, 5H), 3.73 (s, 2H), 3.34 (t, J = 7.3 Hz, 4H), 2.98 (t, J = 7.3 Hz, 4H). |
| 54.6% |
With phosphorus tribromide; In toluene; at 0℃; for 6h;Inert atmosphere; Reflux; |
To a solution of compound O-2 (14.60 g, 74.77 mmol) in toluene (140 mL) was added phosphorus tribromide(21.1 mL, 224.5 mmol) dropwise at 0 C under N2. At the end of the addition, the mixture was refluxed for 6 hours, cooledto rt, quenched with ice water (400 mL) and filtered. The filtrate was washed with NaOH solution and extracted withDCM (100 mL x 3). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuo. Theresidue was purified by silica gel column chromatography (hexane/EtOAc (v/v) = 10/1) to give the title compound 32-1as colorless oil (13.30 g, 54.6%). The compound was characterized by the following spectroscopic data:1H NMR (400 MHz, CDCl3): delta 7.36-7.25 (m, 5H), 3.73 (s, 2H), 3.34 (t, J = 7.3 Hz, 4H), 2.98 (t, J = 7.3 Hz, 4H). |
| 18% |
With phosphorus tribromide; In dichloromethane; at 0 - 20℃; for 8h; |
Into a 500-mL round-bottom flask was placed 2,2'-(benzylazanediyl)diethanol (11.3 g, 57.9 mmol, 1 equiv) and CH2C12 (100 mL). This was followed by the dropwise addition of phosphorus tribromide (34.6 g, 128 mmol, 2.2 equiv) at 0 C. The resulting solution was stirred for 8 h at room temperature. The reaction was then quenched by the addition of 80 mL of ice-water. The pH value of the solution was adjusted to 7 with sat. aq. Na2C03 solution. The resulting solution was extracted with 3x80 mL of CH2C12. The combined organic layers were dried over anhydrous Na2S04, filtered, and concentrated under vacuum. The residue was purified by normal phase chromatography on silica gel using CH2Cl2/petroleum ether (1 :3). The collected fractions were concentrated under vacuum to afford 3.3 g (18% yield) of the title compound as yellow oil. 1H MR (400 MHz, CDC13) delta (ppm): 7.34-7.27 (m, 5H), 3.73 (s, 2H), 3.43 (t, J= 7.2 Hz, 4H), 2.98 (t, J= 7.2 Hz, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping