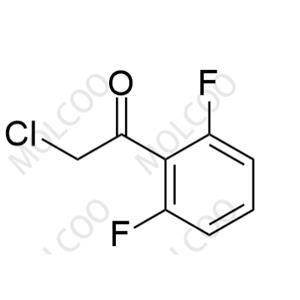
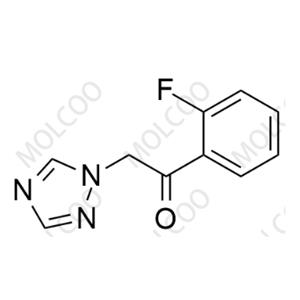
Voriconazole Impurity 41 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 50mg |
| Min. Order: | 10mg |
| Supply Ability: | 10000000 |
| Update Time: | 2025-02-22 |
Product Details
| Product Name: Voriconazole Impurity 41 | CAS No.: 929249-84-3 |
| Min. Order: 10mg | Purity: 98 |
| Supply Ability: 10000000 | Release date: 2025/02/22 |

Voriconazole, as a broad-spectrum antifungal agent, plays a significant role in clinical treatment. However, impurities in the drug may affect its efficacy and safety. Therefore, Voriconazole impurity reference standards play a crucial role in drug research and development, production, and quality control processes.
Product Overview:
Voriconazole impurity reference standards are standardized samples specifically designed for potential impurities in Voriconazole drugs. These reference standards feature high purity, high stability, and high accuracy, enabling qualitative and quantitative analysis of impurities in drugs to ensure compliance with quality standards.
Product Features:
High Purity: The purity of impurity reference standards typically exceeds 98%, ensuring the accuracy of analytical results.
Multiple Specifications: Impurity reference standards are available in various specifications and packaging to meet the needs of different laboratories and analytical methods.
Strict Quality Control: They undergo rigorous quality control processes to ensure compliance with international pharmacopoeia and relevant industry standards.
Ease of Use: Impurity reference standards are easy to dissolve and prepare, facilitating rapid and accurate analysis by laboratory personnel.
Application Scenarios:
Drug Research and Development: In the drug research and development stage, impurity reference standards can be used to assess the content and types of impurities in new drugs, providing data support for drug optimization.
Production Monitoring: During production, impurity reference standards can be used to monitor the content of impurities in drugs, ensuring stable product quality.
Quality Control: In quality control, impurity reference standards can be used for instrument calibration, analytical method validation, and assessment of impurity content in drugs to ensure compliance with quality standards.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-14 | |
| $0.00/1KG |
VIP5Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2024-04-22 | |
| $65.00/1kg |
VIP1Y
|
Shandong Xuhuang New material CoLTD
|
2025-02-21 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake





 China
China