
Umeclidinium Bromide NEW
| Price | Get Latest Price | ||
| Package | 1Kg/Bag | 25Kg/Bag | 100Kg/Bag |
| Min. Order: | 1Kg/Bag |
| Supply Ability: | 20 tons |
| Update Time: | 2025-02-21 |
Product Details
| Product Name: Umeclidinium Bromide | CAS No.: 869113-09-7 |
| EC-No.: 638-761-1 | Min. Order: 1Kg/Bag |
| Purity: 0.99 | Supply Ability: 20 tons |
| Release date: 2025/02/21 |
Product Information
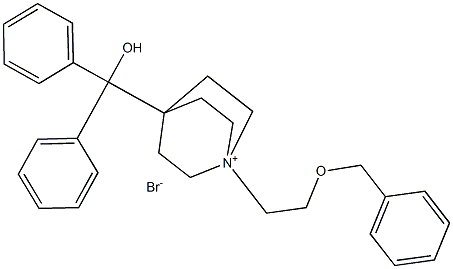
| Product Name: | Umeclidinium bromide |
| Synonyms: | DIPHENYL-[1-(2-PHENYLMETHOXYETHYL)-1-AZONIABICYCLO[2.2.2]OCTAN-4-YL]METHANOL;BROMIDE;CS-1562;Umeclidinium bromide(GSK573719A);1-[2-(Benzyloxy)ethyl]-4-(hydroxydiphenylmethyl)-1-quinuclidinium Bromide;Umeclinidium bromide;GSK573719A;GSK-573719A;GSK 573719A;1-(2-(benzyloxy)ethyl)-4-(hydroxydiphenylmethyl)quinuclidin-1-ium bromide;GSK573719A |
| CAS: | 869113-09-7 |
| MF: | C29H34BrNO2 |
| MW: | 508.48976 |
| EINECS: | 638-761-1 |
| Product Categories: | API;869113-09-7 |
| Mol File: | 869113-09-7.mol |
 | |

Usage

Company Profile Introduction
Sinoway Industrial co., ltd. was established in 1987 in Xiamen, China, and now has been a leading group specialized in research, development, custom manufacturing and trading of pharmaceutical intermediates, APIs, health and food supplements, cosmetic raw materials, herbal extracts, polypeptides and prostaglandin derivatives, etc..
We have passed ISO9001:2015. With qualified products, competitive price and excellent service, we have received great reputation of our customers from all over the world, including Southeast Asia, Europe, North America, South America, Middle East and other countries. We have over 20 years good partners in Japan、Korea and Switzerland.
Sinoway has a professional R&D team. We have built close cooperative relationships with many research institutes and universities in China. Moreover, Sinoway combines market analysis with technology, providing new business trends to our clients.
In addition, we have very close cooperation relationships with many Chinese pharmaceutical factories. These pharmaceutical manufacturers have GMP regulated workshops, advanced production and testing equipments, first-class QC labs and a production team with rich experience. We can supply various Active Pharmaceutical Ingredients and finished products which meet EP/USP/BP standards. Chinese GMP, EU-GMP, COS/CEP certificates with DMF documents are available, and many products have been approved by FDA.
We believe that quality and innovation are vital for an enterprise. Adhering to the business philosophy of integrity and win-win cooperation, Sinoway is looking forward to have a bright future with you together.
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $34.00/1mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $5.00/1KG |
VIP2Y
|
Henan Fengda Chemical Co., Ltd
|
2024-04-16 | |
| $10.00/1kg |
Wuhan Xinhao Biotechnology Co., Ltd
|
2024-03-11 | ||
| $0.00/1kg |
VIP2Y
|
Hebei Kingfiner Technology Development Co.Ltd
|
2023-12-20 | |
| $70.00/1kg |
VIP2Y
|
Zibo Hangyu Biotechnology Development Co., Ltd
|
2023-11-20 | |
| $10.00/1000g |
Anhui Zhongda Biotechnology Co., Ltd
|
2023-10-18 | ||
| $100.00/25kg |
VIP5Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-09-18 | |
| $0.00/25kg |
VIP5Y
|
Hebei Mujin Biotechnology Co.,Ltd
|
2023-06-01 | |
| $0.00/25KG |
PNP Biotech Co. Ltd
|
2022-10-26 | ||
| $10.00/1G |
Lihe Pharm Technology(Wuhan)Co.,Ltd
|
2022-08-04 |
- Since: 1996-07-16
- Address: Fujian China ,16F, Huicheng Comm. Complex, 839 Xiahe RD.
INQUIRY











 China
China