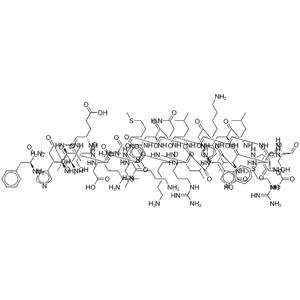
Teriparatide acetate NEW
| Price | $10 | $6 | $2 |
| Package | 1KG | 100KG | 1000KG |
| Min. Order: | 1KG |
| Supply Ability: | g-kg-tons, free sample is available |
| Update Time: | 2024-04-28 |
Product Details
| Product Name: Teriparatide acetate | CAS No.: 52232-67-4 |
| Min. Order: 1KG | Purity: 99% |
| Supply Ability: g-kg-tons, free sample is available | Release date: 2024/04/28 |
| Lead time: In stock | Packaging: bottle/drum/bucket/IBC, as request. |
| Delivery: By sea, by air, by express | Origin: Manufacturer |
1. Product information
| Name | Teriparatide acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Teriparatide is a PHT agonist, with an IC50 of 2 nM in HEK293 cells. Sequence: Ser-Val-Ser-Glu-Ile-Gln-Leu-Met-His-Asn-Leu-Gly-Lys-His-Leu-Asn-Ser-Met-Glu-Arg-Val-Glu-Trp-Leu-Arg-Lys-Lys-Leu-Gln-Asp-Val-His-Asn-Phe. |
|---|---|
| Related Catalog | Signaling Pathways >> Others >> Others Research Areas >> Cancer Peptides |
| Target | IC50: 2 nM (PTH)[1]. |
| In Vivo | Trabecular bone calcium and dry weight of the distal femur increased significantly in Teriparatide-treated animals. The increase in trabecular calcium compared with vehicle control occurred as early as 1 week after initiation of treatment with a 35% and 45% increase, respectively, for 10 μg/kg and 40 μg/kg Teriparatide. Similar results were observed for trabecular dry weight. After 4 weeks of treatment with 10 mg/kg or 40 mg/kg Teriparatide, trabecular calcium increased significantly by 70% and 123%, respectively, compared with the vehicle and by 73%[1]. The 4-week Teriparatide administration increase the pore ratio, number, and density as well as the cortical area, thickness, and bone mineral content (BMC), without significant influencing the volumetric bone mineral density (BMD). The 4-week Teriparatide administration + 8-week vehicle administration decrease the pore ratio, number, and density as well as the cortical area and thickness, compared with the 4-week Teriparatide administration, but the pore ratio, cortical area, and thickness are still higher compared with the 12-week vehicle administration. The 4-week Teriparatide administration + 8-week higherdose IBN administration increase the cortical area, thickness, BMC, and volumetricBMD and decrease the pore ratio, but not the pore number or density, compared with the 4-week Teriparatide administration + 8-week vehicle administration[2]. |
| Animal Admin | Rats[1] Teriparatide is administered daily to 4-week-old male rats for 1, 2, or 4 weeks with different concentrations (10, 40 μg/kg). At each time point, longitudinal growth, expressed as maximal femur length, is not statistically different between treated and control rats or between the two different treatment groups. Midfemur diaphyseal widths also do not differ between groups[1]. Rabbits[2] Forty-two female New Zealand white rabbits (17-21 weeks old) are used throughout the study. After 10 days of adaptation to their new environment, the rabbits (18-22 weeks old) are randomized into six groups of 7 animals each using the stratified weight method, as follows: 4-week vehicle administration group (4W-Veh), 4-week Teriparatide (TPTD) administration group (4W-Teriparatide: 20 μg/kg, subcutaneously [s.c.], daily), 12-week vehicle administration group (12W-Veh), 4-week Teriparatide administration + 8-week vehicle administration group (4W-Teriparatide + 8W-Veh), 4-week Teriparatide administration + 8-week lower-dose IBN administration group (4W-Teriparatide + 8W-IBN(L): 20 μg/kg of IBN, s.c., every 4 weeks), and 4-week Teriparatide administration + 8-week higher-dose IBN administration group (4W-Teriparatide + 8W-IBN(H): 100 μg/kg of IBN, s.c., every 4 weeks)[2]. |
| References | [1]. Frolik CA, et al. Comparison of recombinant human PTH(1-34) (LY333334) with a C-terminally substituted analog of human PTH-related protein(1-34) (RS-66271): In vitro activity and in vivo pharmacological effects in rats. J Bone Miner Res. 1999 Feb;14(2):163-72. [2]. Iwamoto J, et al. Influence of Teriparatide and Ibandronate on Cortical Bone in New Zealand White Rabbits: A HR-QCT Study. Calcif Tissue Int. 2016 Nov;99(5):535-542. |
| Molecular Formula | C181H291N55O51S2 |
|---|---|
| Molecular Weight | 4177.77000 |
| Appearance of Characters | powder |
| Storage condition | ?20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | SQ7770000 |
2. Packaging
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping

4. Contact information
For more details, pls contact us freely.
Mob: 86 17630971917
WhatsApp/Skype/Wechat/LINE: 86 17630971917
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $5.00/1Box |
VIP1Y
|
Hebei Jiafan Trading Company Limited
|
2024-12-31 | |
| $0.00/1Box |
VIP1Y
|
American HealthyMorph LLC
|
2024-12-26 | |
| $0.00/1box |
VIP1Y
|
Shandong Huizhihan Supply Chain Co., Ltd
|
2024-10-31 | |
| $1110.00/1mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-10-23 | |
| $100.00/50kg |
VIP1Y
|
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
|
2024-08-13 | |
| $50.00/1kg |
VIP1Y
|
Shandong Deshang Chemical Co., Ltd.
|
2024-07-09 | |
| $35.00/1box |
Hebei Xinsheng New Material Technology Co., LTD.
|
2024-05-14 | ||
| $0.00/1Gram |
VIP1Y
|
Hangzhou Hyper Chemicals Limited
|
2024-05-09 | |
| $0.00/1g |
VIP1Y
|
Shaanxi TNJONE Pharmaceutical Co., Ltd
|
2024-04-15 | |
| $0.00/1Box |
VIP1Y
|
Shanghai Affida new material science and technology center
|
2024-04-12 |
- Since: 2023-02-10
- Address: Room 01, 2288 E05, Building 14, East Henan University, Science and Technology Park, 279 Xisanhuan Ro




 China
China