Sodium dithionite NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2025-03-21 |
Product Details
| Product Name: Sodium dithionite | CAS No.: 7775-14-6 |
| EC-No.: 231-890-0 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2025/03/21 |
| CAS: | 7775-14-6 |
| MF: | Na2O4S2 |
| MW: | 174.11 |
| EINECS: | 231-890-0 |
| Product Categories: | inorganic chemical raw material;inorganic salt;INORGANIC & ORGANIC CHEMICALS;Inorganics;sulphite;7775-14-6 |
| Mol File: | 7775-14-6.mol |
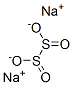 | |
| Sodium dithionite Chemical Properties |
| Melting point | 300 °C |
| Boiling point | 1390°C |
| density | 2.13 |
| Fp | >100°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 250 g/L (20°C) |
| form | Powder/Solid |
| color | White |
| Odor | None or slight scent of sulfur dioxide |
| PH | 5.5-8.5 (50g/l, H2O, 20℃) |
| Water Solubility | 250 g/L (20 oC) |
| Sensitive | Moisture Sensitive |
| Merck | 14,8626 |
| Stability: | Stable, but air sensitive. Incompatible with strong acids, strong oxidizing agents, water, moisture. |
| LogP | -2.756 (est) |
| CAS DataBase Reference | 7775-14-6(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium hydrosulfite (7775-14-6) |
| Sodium dithionite Usage And Synthesis |
| Description | Sodium dithionite is also called sodium hydrosulfite, sodium hydrosulphite, sodium sulfoxylate, and sulfoxylate. Sodium dithionite is not stable under physiological conditions, with the rate of decomposition increasing with increasing acidity. Upon contact with moisture, it is oxidized to hydrogen sulfite (HSO3-), sulfite (SO32-) and hydrogen sulfate (HSO4-). Under strongly acidic conditions it may liberate sulfur dioxide. Under anaerobic conditions (such as in the lower gastrointestinal tract), hydrogen sulfite (HSO3-) and thiosulfate (S2O32-) may be formed. Hydrogen sulfite (HSO3-) can be absorbed after ingestion. It is efficiently metabolized and the major part rapidly is excreted as sulfate into the urine. Sodium dithionite is widely used in industry owing to its reducing properties and ability to react with oxygen. It is used in textile industry for dyeing, in the pulp and paper industry as a reducing bleach to remove yellow discoloration from cellulose based products, as an oxygen scavenger in boilers, in conservation to remove iron stains on cultural artifacts, and in water treatment for controlling iron flash on white fabrics in bleaching environments. It is also used in photographic film, clay, wine, leather goods, foods and beverages, polymers, cleaners, gas purification, environmental remediation, metal recovery, and chemical processing. |
| Uses | Sodium dithionite is a strong reducing agent and is produced commercially for use in vat dyeing. |
| Preparation | The most convenient production method involves a reaction of a suspension of metallic zinc dust with sulphur dioxide to form zinc dithionite, followed by metathesis with sodium carbonate to form the corresponding sodium dithionite: Zn+2SO2->ZnS2O4 ZnS204 + Na2CO3 -> Na2S2O4 + ZnCO3 |
| References | [1] http://chemicalsolutions.net/sodium-hydrosulfite.html [2] http://www.inchem.org/documents/sids/sids/7775146.pdf [3] Lyndsie Selwyn, Season Tse (2008) The chemistry of sodium dithionite and its use in conservation, 53, 61-73 |
| Chemical Properties | White solid |
| Uses | Sodium dithionite is a reducing agent for vat dyes,reducing hair bleaching agents,vat dyes printing auxiliaries, silk scouring and bleaching agents, coloring agents and objects stripping vat cleaning agent, particularly in dyeing with indigo and vat dyes; bleaching soaps, straw; removing dyes from dyed fabrics. |
| Uses | Sodium hydrosulfite is used as a reducing agent in aqueous solutions, sulfonating agent , chelating agent and decolorizing agent in organic reactions. It finds application in water treatment, gas purification, cleaning, leather, polymers, photography, and many others. It is involved in chemical enhanced oil recovery to stabilize polyacrylamide polymers against radical degradation in the presence of iron. It plays an important role to determine the iron content in soil chemistry. |
| Production Methods | An alternative route for dithionite production is the reduction of sodium bisulfite with sodium borohydride. Sodium borohydride is obtained by reacting boron trimethyl ester, B(OCH3)3, with sodium hydride, NaH. The resulting product is hydrolyzed with water, and methanol is evaporated. An alkaline, aqueous solution is obtained, containing about 12% NaBH4 and 40% NaOH. This solution is commercially available. Reaction to dithionite is made on-site by adding sulfur dioxide and some additional caustic soda. Storages, handling and mixing of sulfur dioxide and of the Borol? liquid are not everywhere cost competitive. NaBH4+8NaOH+8SO2 → 4Na2S2O4+NaBO2+6H2O. |
| Preparation | Sodium dithionite is today produced mainly from sodium formate and sulfur dioxide. Zinc dithionite, prepared on-site from sulfur dioxide and zinc dust, was formerly more important than the sodium salt. The heavy metal zinc ended up in the effluent, which caused environmental problems. Today in pulp mills on-site production of zinc dithionite is no longer practiced. The number of plants using sodium amalgam and sulfur dioxide for dithionite production is decreasing with the number of amalgam cells in electrolysis. On-site generation of dithionite from alkaline sodium borohydride solution, sodium bisulfite, and sulfur dioxide is used by some mills. Sodium dithionite is available as crystalline powder (&90% Na2S2O4) or as a refrigerated 150 g/L solution stabilized with alkali. The white crystals can decompose on heating. Low alkalinity, pH8 to 13, stabilizes dithionite solution at low temperature. These solutions should be kept well below 10 °C with the exclusion of air. In presence of air, oxidation rapidly yields sulfate and sulfite. The powder product prepared via the amalgam route dissolves into an alkaline solution as it contains sodium carbonate and sulfite. This can trigger the precipitation of calcium carbonate (water hardness) in the solution tank. Therefore, it is recommended to add small amounts of chelants during the dissolution step. (Chelants are not required to stabilize the bleaching reaction.) Sodium dithionite prepared by the formate route dissolves into a slightly acidic solution. Dithionite powder can ignite if exposed to high humidity or elevated temperature. |
| Definition | ChEBI: An inorganic sodium salt that is the disodium salt of dithionous acid. |
| General Description | Sodium dithionite is a whitish to light yellow crystalline solid having a sulfur dioxide-like odor. Sodium dithionite spontaneously heats on contact with air and moisture. This heat may be sufficient to ignite surrounding combustible materials. Sodium dithionite is soluble in water. Under prolonged exposure to fire or intense heat containers of Sodium dithionite may violently rupture. Sodium dithionite is used in dyeing and to bleach paper pulp. |
| Reactivity Profile | Inorganic reducing agents, such as Sodium dithionite, react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Their reactions with oxidizing agents may be violent. Sulfites and hydrosulfites (dithionites) can react explosively with strong oxidizing agents (sodium chlorite). Sulfites generate gaseous sulfur dioxide in contact with oxidizing acids and nonoxidizing acids. |
| Hazard | Fire risk in contact with moisture. To extin- guish fires, flood the reacting mass with water. |
| Health Hazard | Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution. |
| Fire Hazard | Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated. |
| Flammability and Explosibility | Not classified |
| Safety Profile | Toxic and an irritant. An allergen. Flammable when exposed to heat or flame. Ignites on contact with water or sodium chlorite. To extinpsh fires, flood the reacting mass with water. Decomposes violently when heated to 19OOC and emits toxic fumes of SOx and NazO |
| storage | Sodium dithionite crystals are available in steel containers (1 or 2 tons) or in steel drums (200 kg). Because of the danger of spontaneous ignition in humid air, sodium dithionite must be stored under dry and cool conditions. The sites for the preparation of dithionite solutions must permit handling without risks, high humidity should be avoided and remote fire control should be available. Commercial dithionite products are classified as self-igniting hazardous goods (Class 4.2, UN 1384). Local rules for transportation and storage must be obeyed. |
| Properties and Applications | INDEX/GRADESH-90SH-88SH-85CONTENT90%88%85%Na 2 S 2 O 4≥90%≥88%≥85%Fe≤20ppm≤20ppm≤20ppmZinc (Zn)≤1ppm≤1ppm≤1ppmOther heavy metal(calculated as Pb)≤1ppm≤1ppm≤1ppmWater Insolubles≤0.05%≤0.05%≤0.05%Shelf Life(month)121212 |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $10.00/1KG |
VIP7Y
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2024-11-13 | |
| $5.00/1KG |
VIP4Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-20 | |
| $20.00/1kg |
VIP5Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-10-26 | |
| $100.00/1kg |
Henan Bao Enluo International TradeCo.,LTD
|
2023-07-10 | ||
| $50.00/1KG |
Weijer International Trade (Hebei) Co., Ltd
|
2023-03-16 | ||
| $1075.00/1Tons |
VIP3Y
|
Hebei Dangtong Import and export Co LTD
|
2023-03-06 | |
| $15.00/1KG |
Qiuxian Baitai New Material Co., LTD
|
2021-11-02 | ||
| $10.00/1KG |
Hebei Zhanyao Biotechnology Co. Ltd
|
2021-10-15 | ||
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-08-11 | ||
| $1.00/1BOU/Drum |
HONG KONG IPURE BIOLOGY CO.,LIMITED
|
2021-01-19 |
- Since: 2017-12-08
- Address: 1013, Building B, CR Wanda Plaza, Qiaoxi District, Shijiazhuang City, Hebei Province, China
13288715578
sales@hbmojin.com











 Japan
Japan