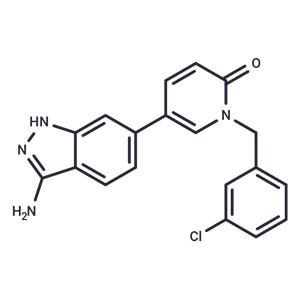
| Name | SLV-2436 |
| Description | SLV-2436 (SEL201-88) is a novel effective and ATP-competitive inhibitor of MNK1 and MNK2 (IC50: 10.8/5.4 nM). |
| Cell Research | Western blot analysis. Cells were treated with dasatinib, imatinib, or SEL201 at the indicated times, and pellets were harvested to obtain protein extracts. Briefly, cell pellets were lysed in RIPA buffer (50 mM Tris-HCl, pH 8.0, with 150 mM sodium chloride, 1.0% Igepal CA-630 [NP-40], 0.5% sodium deoxycholate, and 0.1% SDS). After sonication, cell lysates were centrifuged at 15,871 g for 15 minutes. The supernatants were collected, and protein concentrations were quantified. Equal amounts of protein were loaded and separated on a 10% SDS-PAGE. After transferring to a nitrocellulose membrane (Bio-Rad), 5% milk/TBS was used to block for 1 hour, and then probed for target antibodies overnight at 4°C. After incubation with HRP-conjugated secondary antibodies for 1 hour at room temperature, the signals of targeted protein were developed with chemiluminescence substrate ECL Western blotting detection reagent. |
| Animal Research | All animals were handled in strict accordance with the good animal practice and maintained according to the standards of pathogen-free conditions. The pharmacokinetic profile of SEL201 was assessed in 6-week-old female CD-1 mice (3 animals per time point). SEL201 was freshly dissolved in DMSO and then diluted in Captisol (Ligand) for administration with a volume of 10 μl per 1 g of body weight via the oral (p.o.; 5 mg/kg) or i.v. (2 mg/kg) route. Animals were sacrificed at 8 time points (5, 15, and 30 minutes and 1, 2, 4, 6, and 24 hours) and blood samples harvested. Plasma samples were collected and stored at –80°C for further analysis. To evaluate the pharmacodynamic properties of SEL201, 10- to 16-week-old male C57BL/6 mice (stock 000664, The Jackson Laboratory) were divided into a control group and 3 dosing groups. Animals were given either vehicle (DMSO + N,N-Dimethylacetamide + Captisol) or SEL201 at 10-, 25-, and 50-mg/kg doses (freshly dissolved). Drugs were administered p.o. in a volume of 10 μl per 1 g of body weight. Each animal received a total of 5 doses with the twice-daily schedule (i.e., every 12 hours). Body weight was assessed once daily. Six animals per experimental group supported sample collection at 2-time points (i.e., 4 hours and 24 hours) after the last, fifth administration, with 3 animals per time point. Plasma samples were collected and stored at –80°C for further analysis. For safety assessment of SEL201, 7- to 8-week-old tumor-bearing female Hsd: Athymic Nude-Foxn1nu mice (strain code 069, Envigo) were used. Before use, SEL201 was freshly dissolved, and doses of 50 mg/kg were administered twice daily p.o. in a volume of 10 μl per 1 g of body weight. Body weight was assessed every day. At the end of the experiment on day 37, mice were anesthetized and blood samples for total cell counts and biochemistry were obtained. |
| In vitro | In vitro kinase assays, using recombinant MNK1 and MNK2 protein and increasing concentrations of the SLV-2436 show that SLV-2436 is highly potent, with an IC50 of 10.8 nM and 5.4 nM for MNK1 and MNK2, respectively. To confirm the kinome selectivity of SLV-2436, the broad KINOMEscan (DiscoverX) competitive binding assay at 1 μM is performed, which included 450 distinct kinases (32). The binding profile for SLV-2436 is observed, which was significantly concentrated in the CAMK family of kinases that comprises MNK1 and MNK2. |
| In vivo | To investigate the pharmacodynamic properties of SEL201, mice were administered five consecutive oral doses of 10, 25, and 50 mg/kg every 12 hours (twice-daily schedule). At 10 mg/kg twice daily, 4 hours after the fifth dose, plasma concentration was 125 ng/ml SEL201. Doses of 25 and 50 mg/kg twice daily (50 and 100 mg/kg/day) led to dose-dependent increases in plasma exposure, with average levels of 1,299 ng/ml and 2,075 ng/ml, respectively. At 24 hours, SEL201 was detectable with concentrations of 9, 73, and 124 ng/ml for the 10, 25, and 50 mg/kg twice-daily groups. Oral (p.o.) administration of 50 mg/kg twice daily (100 mg/kg/day) for 37 days was well tolerated, showing no overt clinical toxicity. Blood chemistry, including hematological and biochemical parameters, confirmed safety at 100 mg/kg/day. SEL201 demonstrated good oral bioavailability, achieving a maximum plasma concentration of 1,078 ng/ml within 0.25 hours after p.o. administration. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 50 mg/mL (142.53 mM)
|
| Keywords | MAP kinase interacting kinase | inhibit | SEL201 | MAPK interacting kinase | Mitogen activated protein kinase interacting kinase | SLV-2436 | Inhibitor | SEL 201 | MNK | SLV2436 |
| Inhibitors Related | EB1 | QL-X-138 | QL-X-138 HCl | MK2-IN-3 hydrate | DS12881479 | ETC-206 | Tomivosertib | CGP 57380 | AZ7550 |
| Related Compound Libraries | Anti-Colorectal Cancer Compound Library | Target-Focused Phenotypic Screening Library | Bioactive Compound Library | Pain-Related Compound Library | Kinase Inhibitor Library | Inhibitor Library | Bioactive Compounds Library Max | Anti-Cancer Compound Library | Anti-Cancer Active Compound Library | MAPK Inhibitor Library |



 United States
United States