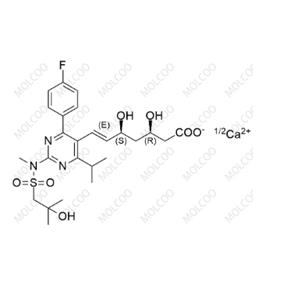
Product Details
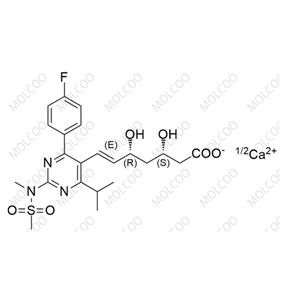
| Product Name: Rosuvastatin EP Impurity A | CAS No.: 1714147-47-3 |
| Min. Order: 10mg | Purity: 98%+ |
| Supply Ability: 20g | Release date: 2025/05/07 |
Professional R&D, Quality Assurance
Our R&D team boasts years of experience in developing Rosuvastatin impurity reference standards, dedicated to providing customers with the highest quality products. Through rigorous quality control testing, we ensure that each batch meets international standards and customer requirements.
Comprehensive Services, Meeting Needs
Beyond providing high-quality Rosuvastatin impurity reference standards, we also offer comprehensive service support. From product consultation, purchasing guidance, to after-sales support, we always put customers first, meeting your various needs.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-04-15 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-24 | |
| $0.00/1KG |
VIP1Y
|
Shaanxi Xianhe Biotech Co., Ltd
|
2025-05-06 | |
| $1.00/1KG |
VIP6Y
|
Career Henan Chemical Co
|
2019-12-25 |




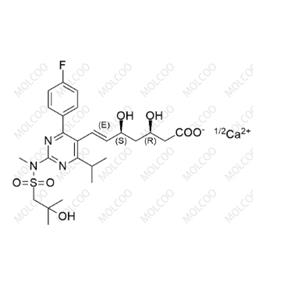
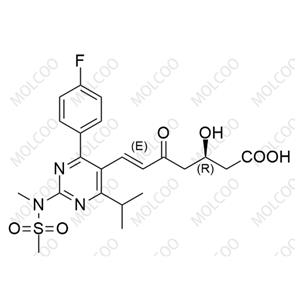

 China
China