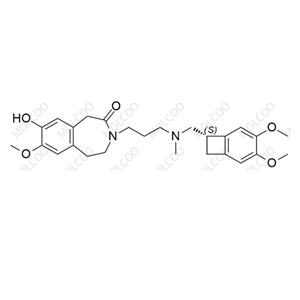
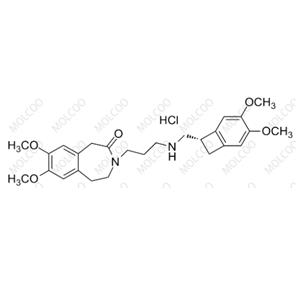
Ivabradine Impurity 34 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-02-22 |
Product Details
| Product Name: Ivabradine Impurity 34 | CAS No.: 304464-97-9 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/02/22 |
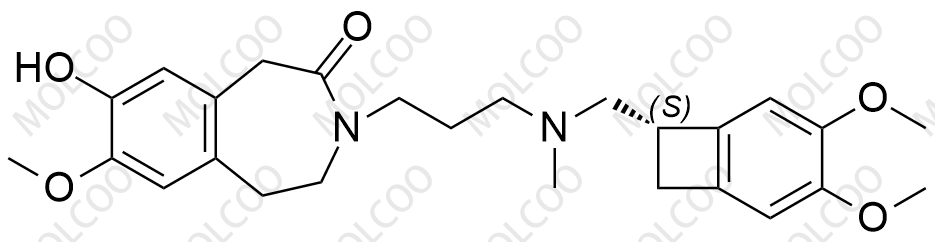
Ivabradine Impurity Reference Standards
Product Details:
Ivabradine, as an important pharmaceutical ingredient, its impurity reference standards play a crucial role in drug research and development, quality control, and production processes. Our Ivabradine impurity reference standards feature high purity, high stability, and precise chemical structures, making them widely applicable in drug analysis, impurity detection, and quality standard formulation.
Product Features:
High Purity: Prepared using advanced manufacturing techniques to ensure a purity level of over 99%.
Accurate Structure: Rigorously synthesized and structurally identified to ensure complete consistency with the target impurity.
Stable and Reliable: Stored under appropriate conditions to maintain stability and reliability over extended periods.
Applications:
Drug Research and Development: Serves as an essential reference material in the drug development process, assessing drug purity and impurity content.
Quality Control: Monitors and controls impurity levels in drugs during production, ensuring drug quality meets standards.
Quality Standard Formulation: Provides accurate impurity reference standards for formulating drug quality standards, ensuring drug safety and efficacy.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $25.00/1kg |
VIP7Y
|
Anhui Dexinjia Biopharm Co., Ltd
|
2024-05-22 | |
| $0.00/10MG |
VIP5Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-10-11 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-23 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake






 China
China