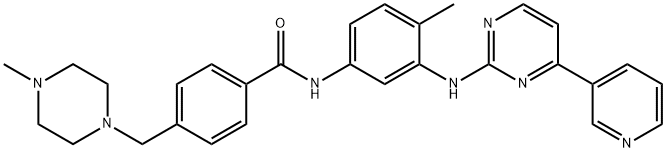
Imatinib NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-09-01 |
Product Details
| Product Name: Imatinib | CAS No.: 152459-95-5 |
| EC-No.: 604-855-6 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/09/01 |
| CAS: | 152459-95-5 |
| MF: | C29H31N7O |
| MW: | 493.6 |
| EINECS: | 604-855-6 |
| Product Categories: | API;Antineoplastic;Aromatics;Heterocycles;GLEVEEC;Anti-cancer & immunity;Molecular Targeted Antineoplastic;Pharmaceutical intermediate;Imatinib;Impurities;Intermediates & Fine Chemicals;Pharmaceuticals;Inhibitors |
| Mol File: | 152459-95-5.mol |
 | |
| Imatinib Chemical Properties |
| Melting point | 208-210°C (dec.) |
| density | 1?+-.0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| pka | pKa1 8.07; pKa2 3.73; pKa3 2.56; pKa4 1.52(at 25℃) |
| form | Solid |
| color | White to Pale Beige |
| Merck | 14,4902 |
| CAS DataBase Reference | 152459-95-5(CAS DataBase Reference) |
| Imatinib Usage And Synthesis |
| Product description | Imatinib is a kind of oral drugs used for the treatment of the chronic myelogenous leukemia (abbreviated CML) of positive symptoms of Philadelphia chromosome (Ber-Abl). It is suitable for being applied to adult patients in acute transformation phase, accelerated phase, and chronic phase with treatment failure with interferon. CML is a kind of hematopoietic stem cell disease caused by the DNA abnormalities in the bone marrow stem cells. DNA abnormalities will produce abnormal proteins and interfere with the normal generation process of the white blood cells in the bone marrow, resulting in the sharp increase in the number of white blood cells. CML is divided into three phases including chronic phase, accelerated phase and crisis phase with the average survival period of the patients in crisis phase being only 2-3 months. Imatinib is also effective in treating the gastrointestinal stromal tumor with the efficiency being about 50%. |
| Approved indications in Europe, the United States and other countries | Novartis's imatinib (imatinib, Glivec) had been approved in Switzerland for being used as first-line drug for the treatment of early-stage adult chronic myelogenous leukemia and can also be applied to patients with various types of chronic granulocytic leukemia. Switzerland is the first countries which had approved to additionally include the above two indications of this drug. On July 28, 2006, the European Agency for the Evaluation of Medicinal Products (EMEA) had recommended the imatinib (Gleevec) of the Novartis Company for the treatment of two new indications-dermatofibrosarcoma protuberans (DFSP) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL). These final approvals of these two indications still need to wait the decision of the European Food and Drug Administration. In addition, Novartis has announced that the application of imatinib in treating hypereosinophilic syndrome and systemic mastocytosis is still in the approval process of FDA and EMEA. The drug has currently been approved in Europe, the United States and other countries for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia (Ph + CML) and gastrointestinal stromal tumors. The above information is edited by the Chemicalbook of Dai Xiongfeng. |
| Precautions | When combined with Ketoconazole, itraconazole, erythromycin, clarithromycin, the Cmax of this drug can (the maximum plasma concentration after the first drug administration) be increased by an average of 26% with the AUC being increased by 40%. The inducers of the metabolizing enzyme of hepatic drugs such as dexamethasone, phenytoin, carbamazepine, rifampicin and barbiturates can significantly reduce blood concentration of the drug. When the CYP3A4 metabolized substrates such as simvastatin, cyclosporine, pimozide, etc. were used in combined with imatinib, the plasma concentration of those drugs can be increased due to its competing with the drug enzyme. |
| Uses | Imatinib can be used for the treatment of the chronic myelogenous leukemia (abbreviated CML) of positive symptoms of Philadelphia chromosome (Ber-Abl). It is suitable for being applied to adult patients in acute transformation phase, accelerated phase, and chronic phase with treatment failure with interferon. |
| Chemical Properties | Orange Solid |
| Uses | Imatinib Mesylate is orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM, respectively. Imatinib also known as Gleevec, Glivec, CGP-57148B, STI-571 & Imatinib |
| Uses | antineoplastic |
| Uses | atypical antipsychotic |
| Uses | Imatinib impurity. |
| Definition | ChEBI: Imatinib is a benzamide obtained by formal condensation of the carboxy group of 4-[(4-methylpiperazin-1-yl)methyl]benzoic acid with the primary aromatic amino group of 4-methyl-N(3)-[4-(pyridin-3-yl)pyrimidin-2-yl]benzene-1,3-diamine. Used (as its mesylate salt) for treatment of chronic myelogenous leukemia and gastrointestinal stromal tumours. It has a role as an apoptosis inducer, a tyrosine kinase inhibitor and an antineoplastic agent. It is a N-methylpiperazine, a member of pyridines, a member of benzamides, an aromatic amine and a member of pyrimidines. It is functionally related to a benzamide. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP1Y
|
Moxin Chemicals
|
2024-12-10 | |
| $0.00/25kg |
VIP3Y
|
Shanghai Joiny Pharmaceutical Co.,LTD
|
2024-12-04 | |
| $10.00/1KG |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2024-11-28 | |
| $79.00/500mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $0.00/25KG |
VIP1Y
|
Shaanxi Dideu New Materials Co. Ltd
|
2024-11-13 | |
| $0.00/1kg |
VIP3Y
|
HangZhou RunYan Pharma Technology Co.,LTD.
|
2024-09-11 | |
| $19.10/1KG |
VIP4Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-20 | |
| $999.00/10kg |
VIP1Y
|
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
|
2024-08-08 | |
| $0.00/1Gram |
VIP1Y
|
Hangzhou Hyper Chemicals Limited
|
2024-05-09 | |
| $8.00/1KG |
VIP2Y
|
Henan Fengda Chemical Co., Ltd
|
2024-03-28 |











 Japan
Japan