
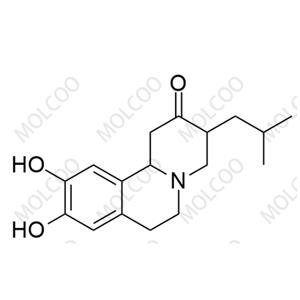
Iloperidone Impurity 27 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 10000 |
| Update Time: | 2025-04-29 |
Product Details
| Product Name: Iloperidone Impurity 27 | CAS No.: 84163-64-4 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 10000 | Release date: 2025/04/29 |

Product Name: Iloperidone Impurity Reference Standard
CAS No.: Including 133454-47-4 (Iloperidone API), 129938-20-1 (Impurity A), 531524-17-1 (Impurity 13), etc.
Molecular Formula & MW: Varies by impurity type (e.g., C12H13F2NO for HC102-201801, MW 225.23; C24H27FN2O4 for Impurity A, MW 426.4806).
Storage Conditions: 2-8°C (some impurities require -20°C storage).
Purity: ≥95% (some ≥98%).
Product Category: Pharmaceutical Impurity Reference Standard.
Applications: Quality control, stability studies, method development, and regulatory filing for Iloperidone API and formulations.
Key Features
Multiple Specifications: Available in 10mg, 25mg, 50mg, and 100mg packages.
Structural Confirmation: Includes COA, HPLC, NMR, and MS spectra for impurity identification.
Regulatory Compliance: Meets ICH, FDA, and NMPA requirements for drug development.
Custom Synthesis: Tailored impurity production for specific research needs.
Applications
Method Development: Validates HPLC and LC-MS methods for impurity separation.
Stability Studies: Evaluates degradation products under various conditions.
Impurity Profiling: Identifies impurity sources to optimize manufacturing processes.
Regulatory Filings: Supports pharmacopeial method validation and impurity limit setting.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $40.00/25mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2025-04-27 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2024-12-20 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-04-01 |







 China
China