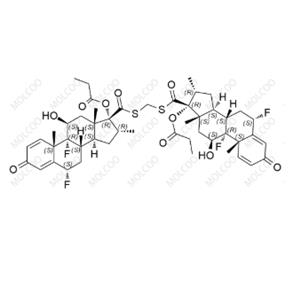
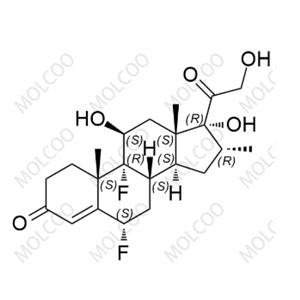
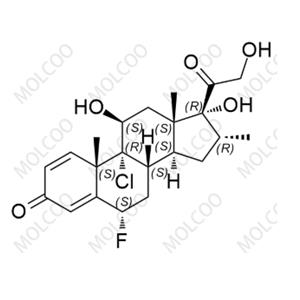
Fluticasone Propionate Impurity 24 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-02-13 |
Product Details
| Product Name: Fluticasone Propionate Impurity 24 | CAS No.: 192191-51-8 |
| Min. Order: 10mg | Purity: 98%+ |
| Supply Ability: 100000 | Release date: 2025/02/13 |
Fluticasone Propionate Impurity Reference Standard
I. Product Overview
The Fluticasone Propionate Impurity Reference Standard serves as an essential reference material for drug development and quality control. We offer a comprehensive range of Fluticasone Propionate impurity reference standards, including but not limited to the following major impurities:
Fluticasone Propionate Impurity A
Fluticasone Propionate Impurity J
Other specific impurities (customized according to customer needs)
II. Product Details
Chinese Name: Fluticasone Propionate Impurity Reference Standard
English Name: Fluticasone Propionate Impurity Reference Standard
CAS Number: Varies according to the specific impurity type (e.g., CAS No. 65429-42-7 for Fluticasone Propionate Impurity A and CAS No. 28416-82-2 for Fluticasone Propionate Impurity J)
Origin: China
Storage Conditions: Store at low temperature to ensure product stability
Available Services: Customized services are available according to customer needs. We also provide basic detection data such as COA (Certificate of Analysis), HPLC (High-Performance Liquid Chromatography), H-NMR (Proton Nuclear Magnetic Resonance), and MS (Mass Spectrometry). Customers can also choose to detect C-NMR (Carbon Nuclear Magnetic Resonance), infrared, ultraviolet, moisture data, quantitative NMR, two-dimensional spectroscopy, etc., according to their own needs.
III. Application Areas
The Fluticasone Propionate Impurity Reference Standard is widely used in drug development, quality control, pharmacokinetic studies, and other fields, providing researchers with accurate and reliable impurity reference standards.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-06 | |
| $0.00/10mg |
VIP1Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-06-29 | |
| $0.00/1g |
VIP2Y
|
Wuhan Cell Pharmaceutical Co., Ltd
|
2023-05-17 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake




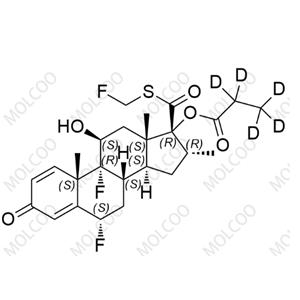

 China
China