
Captopril NEW
| Price | $35 | $1.2 |
| Package | 1kg | 1000kg |
| Min. Order: | 1kg |
| Supply Ability: | g-kg-tons, free sample is available |
| Update Time: | 2024-04-18 |
Product Details
| Product Name: Captopril | CAS No.: 62571-86-2 |
| Min. Order: 1kg | Purity: 99% |
| Supply Ability: g-kg-tons, free sample is available | Release date: 2024/04/18 |
| Lead time: In stock, ready for shipment | Packaging: bag/bottle/drum/IBC |
| Delivery: By express, by air, by sea | Origin: Manufacturer, advantage product |
| COA, MSDS: Available, contact us for details | Name: Mia |
1. Materials information
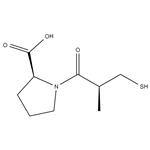
| Name | captopril |
|---|---|
| Synonym | More Synonyms |
| Description | Captopril is a potent, competitive inhibitor of angiotensin-converting enzyme (ACE).Target: ACECaptopril has been shown to have similar morbidity and mortality benefits to those of diuretics and beta-blockers in hypertensive patients. Captopril has been shown to delay the progression of diabetic nephropathy, and enalapril and lisinopril prevent the development of nephropathy in normoalbuminuric patients with diabetes [1]. an equimolar ratio of the cis and trans states of captopril exists in solution and that the enzyme selects only the trans state of the inhibitor that presents architectural and stereoelectronic complementarity with its substrate binding groove [2]. |
|---|---|
| Related Catalog | Signaling Pathways >> Metabolic Enzyme/Protease >> Angiotensin-converting Enzyme (ACE) Research Areas >> Neurological Disease |
| References | [1]. Tzakos, A.G., et al., The molecular basis for the selection of captopril cis and trans conformations by angiotensin I converting enzyme. Bioorg Med Chem Lett, 2006. 16(19): p. 5084-7. [2]. Song, J.C. and C.M. White, Clinical pharmacokinetics and selective pharmacodynamics of new angiotensin converting enzyme inhibitors: an update. Clin Pharmacokinet, 2002. 41(3): p. 207-24. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 427.0±40.0 °C at 760 mmHg |
| Melting Point | 104-108 °C(lit.) |
| Molecular Formula | C9H15NO3S |
| Molecular Weight | 217.285 |
| Flash Point | 212.1±27.3 °C |
| Exact Mass | 217.077271 |
| PSA | 96.41000 |
| LogP | 0.27 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.551 |
| Water Solubility | soluble |
Captopril MSDS(Chinese) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |   GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H361 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S36/37-S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | UY0550000 |
| HS Code | 2933990090 |
2. Packaging of materials
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping & Delivery
By Express
Provide door to door service
Suitable for goods under 50kg
Delivery: 3-7 days
Cost: low cost

By Air
Provide airport to airport service
Suitable for goods over 50kg
Delivery: 3-14 days
Cost: high cost

By Sea
Provide seaport to seaport service
Suitable for goods over 100kg
Delivery: 2-45 days
Cost: low cost

4. Contact information
For more details, pls contact us freely.
Email address: mia@fdachem.com
Mob: 86 18336764634
WhatsApp/Skype/Wechat/LINE: 86 18336764634
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $48.00/100mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $48.00/100mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $10.00/1KG |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2024-11-19 | |
| $5.00/1KG |
VIP4Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-20 | |
| $0.00/25kg |
VIP1Y
|
Hebei Mojin Biotechnology Co.,Ltd
|
2024-07-25 | |
| $0.00/1kg |
VIP1Y
|
Shaanxi TNJONE Pharmaceutical Co., Ltd
|
2024-05-10 | |
| $80.00/1KG |
Anhui Yisheng Technology Co., LTD
|
2023-01-12 | ||
| $80.00/1KG |
XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD
|
2021-12-29 | ||
| $100.00/1KG |
Qiuxian Baitai New Material Co., LTD
|
2021-11-26 | ||
| $350.00/1Kg/Bag |
VIP5Y
|
Hebei Mujin Biotechnology Co.,Ltd
|
2021-10-08 |
- Since: 2023-02-10
- Address: Room 01, 2288 E05, Building 14, East Henan University, Science and Technology Park, 279 Xisanhuan Ro






 China
China