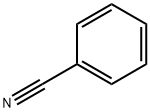
Benzonitrile NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-09-14 |
Product Details
| Product Name: Benzonitrile | CAS No.: 100-47-0 |
| EC-No.: 202-855-7 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/09/14 |
| CAS: | 100-47-0 |
| MF: | C7H5N |
| MW: | 103.12 |
| EINECS: | 202-855-7 |
| Product Categories: | Organics;Sure/Seal Bottles;ACS and Reagent Grade Solvents;Carbon Steel Cans with NPT Threads;ReagentPlus;ReagentPlus Solvent Grade Products;Industrial/Fine Chemicals;Intermediates;Semi-Bulk Solvents;Solvent Bottles;Solvent by Application;Solvent Packaging Options;Solvents;Anhydrous Solvents |
| Mol File: | 100-47-0.mol |
 | |
| Benzonitrile Chemical Properties |
| Melting point | -13 °C (lit.) |
| Boiling point | 191 °C (lit.) |
| density | 1.01 |
| vapor density | 3.6 (vs air) |
| vapor pressure | 1 hPa at 20 °C |
| refractive index | n |
| Fp | 161 °F |
| storage temp. | Store below +30°C. |
| solubility | 10g/l |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Relative polarity | 0.333 |
| Odor | Almond-like. |
| explosive limit | 1.4-7.2%(V) |
| Water Solubility | 10 g/L (100 oC) |
| λmax | λ: 300 nm Amax: 1.0 λ: 310 nm Amax: 0.40 λ: 335 nm Amax: 0.03 λ: 360-400 nm Amax: 0.01 |
| Merck | 14,1097 |
| BRN | 506893 |
| Exposure limits | NIOSH: IDLH 14 ppm(25 mg/m3) |
| Stability: | Stable. Incompatible with strong bases, strong acids, strong oxidizing agents, strong reducing agents. Air-sensitive. Combustible. |
| InChIKey | JFDZBHWFFUWGJE-UHFFFAOYSA-N |
| LogP | 1.5 at 20℃ |
| CAS DataBase Reference | 100-47-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzonitrile(100-47-0) |
| EPA Substance Registry System | Benzonitrile (100-47-0) |
| Benzonitrile Usage And Synthesis |
| Chemical Properties | Bezonitrile is a colorless, oily liquid. It has a almond odor and a bitter taste. Slightly soluble in cold water, the solubility in water at 100°C is 1%; miscible with common organic solvents. When heated to decomposition, benzonitrile emits toxic hydrogen cyanide and oxides of nitrogen (HSDB 1988). |
| Occurrence | Benzonitrile is reported to be found in natural cocoa aroma), in milk products, roasted filberts and peanuts and cooked trassi . Benzonitrile also has been detected in the thermal decomposition products of flexible polyurethane foam. |
| Uses | The most important commercial use for benzonitrile is the synthesis of benzoguanamine, which is a derivative of melamine and is used in protective coatings and molding resins. It is used intermediate for rubber chemicals; solvent for nitrile rubber, specialty lacquers, and many resins and polymers, and for many anhydrous metallic salts. |
| Application | Benzonitrile is a widely utilized as a solvent and an intermediate in industries making drugs, perfumes, dyes, rubber, textiles, resins and specialty lacquers. It finds application as a versatile precursor for many derivatives. It coordinates with transition metal to form complexes which act as synthetic intermediates. |
| Preparation | Benzonitrile can be prepared by following methods: 1) on a small scale by the dehydration in an inert solvent with phosphorus oxychloride or benzenesulfonyl chloride and an organic amine. 2) from benzoic acid by heating with lead thiocyanate. 3) by heating sodium benzenesulfonate with sodium cyanide. 4) by adding benzenediazonium chloride solution to a hot aq sodium cyanide solution containing cupric sulfate and distilling by ammoxidation of toluene. Benzonitrile can also be produced in high yield by the vapor-phase catalytic ammoxidation of toluene. |
| Definition | ChEBI: Benzonitrile is a nitrile that is hydrogen cyanide in which the hydrogen has been replaced by a phenyl group. It is a member of benzenes and a nitrile. |
| Synthesis Reference(s) | Chemistry Letters, 13, p. 1913, 1984 Journal of the American Chemical Society, 111, p. 4903, 1989 DOI: 10.1021/ja00195a050 Tetrahedron Letters, 11, p. 2085, 1970 |
| General Description | Benzonitrile appears as a clear colorless liquid with an almond-like odor. Flash point 161°F. Denser (at 8.4 lb / gal) than water and slightly soluble in water. Used as a specialty solvent and to make other chemicals. |
| Air & Water Reactions | Slightly soluble in water. |
| Reactivity Profile | The cyano group can be readily hydrolyzed in the presence of mineral acids to produce stable, moderately toxic benzoic acid . When heated to decomposition, Benzonitrile emits highly toxic fumes of nitrogen oxides and hydrogen cyanide [Sax, 9th ed., 1996, p. 353]. |
| Hazard | High toxicity; absorbed by skin. |
| Health Hazard | Benzonitrile may enter the human body by ingestion, absorption through the skin, or inhalation. The earliest symptoms of cyano compound intoxication may be weakness, headaches, confusion, and occasionally nausea and vomiting. The respiratory rate and depth will usually be increased at the beginning and at later stages become slow and gasping. Blood pressure is usually normal, especially in the mild or moderately severe cases, although the pulse rate is usually more rapid than normal. |
| Fire Hazard | Special Hazards of Combustion Products: Toxic hydrogen cyanide and oxides of nitrogen may form in fire. |
| Industrial uses | Benzonitrile is used as an intermediate for rubber chemicals and as a solvent for nitrile rubber, specialty lacquers, many resins, polymers and for many anhydrous metallic salts (HSDB 1988; Hawley 1981). It is principally used as an intermediate for benzoguanamine (HSDB 1988). It is also used as an additive in nickel-plating baths, separating naphthalene and alkylnaphthalenes from non-aromatics by azetropic distillation; as jet-fuel additive; in cotton bleaching baths; as a drying additive for acrylic fibers; and in the removal of titanium tetrachloride and vanadium oxychloride from silicon tetrachloride (HSDB 1988; Smiley 1981). Benzonitrile is also used in perfumes at a maximum level of 0.2% in the final product (Opdyke 1979). |
| Safety Profile | Poison by intraperitoneal andsubcutaneous routes. Moderately toxic by ingestion,inhalation, and skin contact. A skinirritant. Combustible liquid. When heated todecomposition it emits toxic fumes of CN- and NOx. |
| Metabolism | Benzonitrile is mainly hydroxylated in vivo to cyanophenols, a small amount being hydrolysed to benzoic acid (Williams 1959). Benzonitrile also forms 6>-hydroxybenzonitrile, m-hydroxybenzonitrile, and /p-hydroxybenzonitrile in rabbits (HSDB 1988). In rabbit, 50% of a dose of 150 mg/kg was converted to conjugated cyanophenols and 10% of the benzonitrile fed was excreted as benzoic acid. Hydrogen cyanide is not a metabolite of benzonitrile (Williams 1959) and cyanide was not found to be formed by benzonitrile either in vivo or in vitro (Tanii and Hashimoto 1984). The in vivo microsomal hydroxylation of specifically deuterated benzonitrile in the rat yielded mainly 4-hydroxybenzonitrile with 41% retention of deuterium (Daly et al 1968). |
| Shipping | UN2224 Benzonitrile, Hazard Class: 6.1; Labels: 6.1—Poisonous materials. |
| Purification Methods | Dry benzonitrile with CaSO4, CaCl2, MgSO4 or K2CO3, and distil it from P2O5 in an all-glass apparatus, under reduced pressure (b 69o/10mm), collecting the middle fraction. Distillation from CaH2 causes some decomposition of benzonitrile. Isonitriles can be removed by preliminary treatment with conc HCl until the odour of isonitrile (carbylamine) has gone, followed by preliminary drying with K2CO3. (This treatment also removes amines.) Steam distil (to remove small quantities of carbylamine). The distillate is extracted into ether, washed with dilute Na2CO3, dried overnight with CaCl2, and the ether is removed by evaporation. The residue is distilled at 40mm (b 96o) [Kice et al. J Am Chem Soc 82 834 1960]. Conductivity grade benzonitrile (specific conductance 2 x 10-8 mho) is obtained by treatment with anhydrous AlCl3, followed by rapid distillation at 40-50o under vacuum. After washing with alkali and drying with CaCl2, the distillate is redistilled in a vacuum several times at 35o before fractionally crystallising several times by partial freezing. It is dried over finely divided activated alumina from which it is withdrawn when required [Van Dyke & Harrison J Am Chem Soc 73 402 1951]. [Beilstein 9 IV 892.] |
| Incompatibilities | Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. They are incompatible Benzonitrile with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. |
| Waste Disposal | (1) Mix with calcium hypochlorite and flush to sewer with water or (2) incinerate. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $1.00/200KG |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2024-10-24 | |
| $0.00/200kg |
VIP3Y
|
Wuhan Biet Co., Ltd.
|
2023-12-18 | |
| $1.50/1g |
VIP3Y
|
Shaanxi Didu New Materials Co. Ltd
|
2022-05-20 | |
| $10.00/1KG |
Hebei baicao biology science and technology co., ltd
|
2022-04-18 | ||
| $0.00/200Kg/Drum |
YANGZHOU SHUANGDING CHEM CO., LTD
|
2020-11-13 | ||
| $1.00/1KG |
VIP7Y
|
Career Henan Chemical Co
|
2018-08-07 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 Japan
Japan