
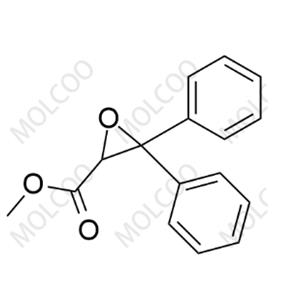
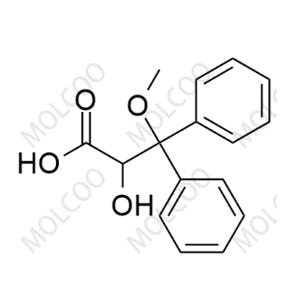
Ambrisentan Impurity NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-04-27 |
Product Details
| Product Name: Ambrisentan Impurity | CAS No.: 178306-47-3 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/04/27 |

Ambrisentan Impurity Reference Standard
Utilized in pharmaceutical R&D, quality control, and regulatory submissions as an analytical standard to detect and quantify impurities in drug substances, ensuring drug safety and efficacy.
High Purity Assurance:Synthesized and purified under rigorous protocols to ensure single-component impurities.
Regulatory Compliance:Adheres to USP, EP, BP pharmacopeia standards, supporting global regulatory filings.
Stability:Stable for up to 3 years when stored at -20°C.
Storage:Store at -20°C, protected from light and moisture.
Shipping:Transported under cryogenic conditions (dry ice or cold chain) to maintain stability.
application area :
Impurity profiling in new drug development
Process optimization and quality control in drug manufacturing
Generic drug bioequivalence studies

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1g |
VIP4Y
|
WUHAN FORTUNA CHEMICAL CO., LTD
|
2022-12-29 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 | ||
| $1.00/1kg |
VIP7Y
|
Career Henan Chemical Co
|
2018-12-20 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-08 |







 China
China