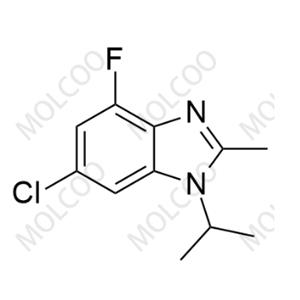
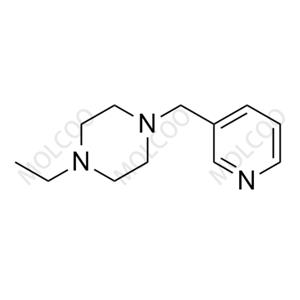
Abemaciclib Impurity 33 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 10000 |
| Update Time: | 2025-03-06 |
Product Details
| Product Name: Abemaciclib Impurity 33 | CAS No.: 2768138-79-8 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 10000 | Release date: 2025/03/06 |

Abemaciclib Impurity Reference Standards
Abemaciclib is an oral selective inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), primarily used for the treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+/HER2-) locally advanced or metastatic breast cancer, as well as adjuvant therapy for early breast cancer. To ensure the quality of Abemaciclib, researchers and pharmaceutical companies require high-quality impurity reference standards for quality control and research and development.
We offer a range of Abemaciclib impurity reference standards, including impurities such as Abemaciclib Impurity C and Abemaciclib Impurity D. These impurity reference standards have undergone rigorous purification and identification processes, with purities exceeding 95%, making them suitable for various applications such as project approval, drug development, and quality control.
Our Abemaciclib impurity reference standards feature the following characteristics:
High Purity: All impurity reference standards have purities above 95%, ensuring the accuracy of results.
Multiple Specifications: We offer various packaging specifications, including 10mg, 25mg, 50mg, and 100mg, to meet different needs.
Strict Quality Control: Each batch of impurity reference standards undergoes rigorous quality control, including the provision of Certificates of Analysis (COA), Nuclear Magnetic Resonance (NMR), High-Performance Liquid Chromatography (HPLC), and Mass Spectrometry (MS) spectra, to ensure product quality.
Welcome inquiries and orders from researchers and pharmaceutical companies!

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1kg |
VIP5Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-10-11 | |
| $1.00/1kg |
VIP1Y
|
S&Y Biochem Co.,Ltd
|
2024-10-14 | |
| $0.00/1g |
VIP1Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-06-28 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake




 China
China