
Cyclopentane
| Price | $1 |
| Package | 1kg |
| Min. Order: | 1g |
| Supply Ability: | 100KG |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Cyclopentane | CAS No.: 287-92-3 |
| Min. Order: 1g | Purity: 99% |
| Supply Ability: 100KG | Release date: 2019/07/06 |
| PRODUCT:: AD68 |
| Cyclopentane Basic information |
| Physical and chemical properties Cycloalkane Dangerous situation Harmful effects and symptoms of poisoning Protective measures Medical care Transportation requirements Fire extinguishing agent Recommended waste disposal methods Chemical properties Uses Production method Hazards & Safety Information |
| Product Name: | Cyclopentane |
| Synonyms: | Cyclopentan;PENTAMETHYLENE;CYCLOPENTANE;cyclopentane B&J brand 4 L;CYCLOPENTANE, FOR UV-SPECTROSCOPY;CYCLOPENTANE, HPLC GRADE;CYCLOPENTANE, ANHYDROUS;CYCLOPENTANE OEKANAL |
| CAS: | 287-92-3 |
| MF: | C5H10 |
| MW: | 70.13 |
| EINECS: | 206-016-6 |
| Product Categories: | ACS and Reagent Grade Solvents;Amber Glass Bottles;ReagentPlus;ReagentPlus Solvent Grade Products;Solvent Bottles;Solvent by Application;Solvent Packaging Options;Analytical Reagents;Analytical/Chromatography;CHROMASOLV for HPLC;Chromatography Reagents &;HPLC &;HPLC Grade Solvents (CHROMASOLV);HPLC/UHPLC Solvents (CHROMASOLV);UHPLC Solvents (CHROMASOLV);Pharmaceutical Intermediates;Organics;Organic Chemicals;Alpha Sort;C;CAlphabetic;Chemical Class;CO - CZChemical Class;Hydrocarbons;NeatsAnalytical Standards;Volatiles/ Semivolatiles;Solvents - HPLC;Analytical Reagents;Chromatography/CE Reagents;Anhydrous Grade SolventsSolvents;Solvent Bottles;Solvents;Sure/Seal? Bottles;ReagentPlus(R) Solvent Grade Products;ReagentPlus(R)Solvents;Reagent Grade Solvents;ReagentSolvents;Amber Glass Bottles;CHROMASOLV for HPLCSolvents;CHROMASOLV Solvents (HPLC, LC-MS);CHROMASOLV(R) HPLC Grade Solvents |
| Mol File: | 287-92-3.mol |
 |
|
| Cyclopentane Chemical Properties |
| Melting point | -94 °C |
| Boiling point | 50 °C(lit.) |
| density | 0.751 g/mL at 25 °C(lit.) |
| vapor density | ~2 (vs air) |
| vapor pressure | 18.93 psi ( 55 °C) |
| refractive index | n |
| Fp | −35 °F |
| storage temp. | Flammables area |
| solubility | 0.156g/l insoluble |
| form | Powder |
| color | White |
| explosive limit | 1.5-8.7%(V) |
| Water Solubility | Miscible with ethanol, ether and acetone. Slightly miscible with water. |
| Merck | 14,2741 |
| BRN | 1900195 |
| Stability: | Stable. Highly flammable. Note low flash point and wide explosion limits. Incompatible with strong oxidizing agents. Floats on water, so water is of limited value in putting out fires involving this material. |
| CAS DataBase Reference | 287-92-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclopentane(287-92-3) |
| EPA Substance Registry System | Cyclopentane(287-92-3) |
| Safety Information |
| Hazard Codes | F |
| Risk Statements | 11-52/53 |
| Safety Statements | 9-16-29-33-61 |
| RIDADR | UN 1146 3/PG 2 |
| WGK Germany | 1 |
| RTECS | GY2390000 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | II |
| Hazardous Substances Data | 287-92-3(Hazardous Substances Data) |
| Toxicity | LC (2 hr in air) in mice: 110 mg/l (Lazarew) |
| MSDS Information |
| Provider | Language |
|---|---|
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Cyclopentane Usage And Synthesis |
| Physical and chemical properties | Cyclopentane, also known as "pentamethylene", is a kind of cycloalkane with the formula of C5H10. It has a molecular weight of 70.13. It exists as a kind of flammable liquid. It has a melting point-94.4 °C, boiling point of 49.3 °C, relative density of 0.7460 and the refractive index of 1.4068. It is soluble in alcohol, ether and hydrocarbons and is not soluble in water. Cyclopentane is not a planar ring and has two conformations: envelope conformations and semi-chair conformations. The carbon-carbon-carbon bond angle is close to 109 ° 28 ' with the molecular tension not big and the ring being relatively stable. It has a similar chemical property as alkanes. The lethal concentration in the air for the rats was 3.8 × 10-2. It exhibits red yellow color when having reaction with fuming sulfuric acid while generating nitro cyclopentane and glutaric acid through reaction with nitric acid. Method: it can be obtained from the petroleum ether distillate, through high-pressure cracking on the cyclohexane in the presence of aluminum or catalytic hydrogenation of cyclopentene and cyclopentadiene. Purposes: mainly used as a solvent.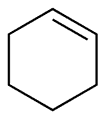 Figure 1 the cyclopentane structure. |
| Cycloalkane | Cycloalkanes are saturated hydrocarbons in which the carbon atoms in the molecule are arranged in a ring and a sufficient number of hydrogen atoms are combined. Cycloalkanes presented in petroleum are mainly cyclopentane and cyclohexane. Cycloalkanes have a higher melting point, boiling point, and relative density than the corresponding straight-chain alkanes. We can use the naphthenic aromatic crude oil to produce high-octane straight-run gasoline with its anti-explosion being better than normal paraffin. Low sulfur-containing paraffin naphthenic crude oil, is not only easy to process, but also an excellent raw material for the production of advanced lubricants. Petroleum containing relative many polycyclic long side chain naphthenic compounds is an ideal material for high-quality lubricants. At room temperature and atmospheric pressure, cycloalkane containing four or less carbon atoms is in the gas form with those contain more than four carbons existing in the liquid form. The cyclopropane and cyclobutane appear as gas, cyclopentane to cycloundecane appears as liquid; cyclododecane and above appears as solid. The chemical nature of the cycloalkane is related to the number of carbon atoms forming the ring. It is referred three-membered ring and four-membered ring, as small ring; the five-to-seven-membered ring as normal ring; the eight-eleven ring as normal ring; the twelve-membered ring and above as large ring. The nuclei lines of carbon nuclei in small rings are not consistent with the axis of bonding orbital. In cyclopropane, the ring formed by the nucleus lines of carbon atoms is an equilateral triangle with each angle being 60° while the angle of the sp3 hybrid orbital axis of carbon-carbon single bonds formed by each carbon 104° (see figure 2 below). Therefore, the orbit has failed to achieve the greatest degree of overlap, causing a large angle tension. Cyclobutane also has angular tension, but being smaller. This leads to the poor stability of the small ring, causing its similar chemical property to olefins that can have ring-opening addition reaction with many reagents. Other ring has less of no angular tension. Cycloalkane and alkanes have similar chemical properties, less prone to have ring-opening reaction such as having reaction with hydrogen. Cyclohexane and higher cycloalkane is more difficult to undergo hydrogenation. 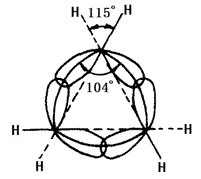 Figure 2 is a schematic representation of sp3 hybridization orbital overlap in cyclopropane. Cyclopropane (at room temperature) and cyclobutane (at the heating conditions) can have addition reaction with halogen and hydrogen halide. The open-loop occurs between the two atoms connecting the most and least numbers of hydrogen. The addition satisfies the Markovian rule. While the normal ring, under the stimulation of the light or heat can have substitution reaction with the halogen. At room temperature, cycloalkane can’t be oxidized by potassium permanganate. Cyclopentane, cyclohexane and its alkyl substituted products are presented in certain petroleum oils. Cycloalkanes may also be synthesized by suitable methods, such as dihaloalkane cyclization and hydrogenation of aromatic hydrocarbons. This information was edited by Xiaonan from Chemicalbook (2015-08-17). |
| Dangerous situation | Ingestion and inhalation are moderately toxic. (2) Being flammable with greater risk of combustion. The allowable concentration in air is 600ppm (1720mg/m3) in the United States. |
| Harmful effects and symptoms of poisoning | Inhalation of high concentrations of cyclopentane can cause central nervous system inhibition, although its acute toxicity is low. Symptoms of acute exposure include excitement at first, followed by the emergence of balance disorders, and even stupor, coma. There are rarely cases of death due to respiratory failure. It has been reported that animals fed with this goods can get severe diarrhea, leading to heart, lung and liver vascular collapse and brain degeneration. |
| Protective measures | It can be used for improving the production equipment. Use skin protective creams or gloves to protect the skin. |
| Medical care | Upon regular physical examination, pay attention to the potential irritation effect of skin and respiratory tract as well as any complications of kidney and liver. |
| Transportation requirements | Grade I flammable liquid. Code of Hazard Regulations: 61013. The container shall be marked with a "flammable liquid" mark on transport. |
| Fire extinguishing agent | See “cyclohexane”. |
| Recommended waste disposal methods | Incineration; |
| Chemical properties | It appears as colorless liquid with a melting point of-93.9 °C, the boiling point of 49.26 ° C, the relative density of 0.7460 (20/4 ° C), the refractive index of 1.4068 and the flash point of-37 °C. It is miscible with alcohol, ether and other organic solvents, being not be easy to be dissolved in water. |
| Uses | (1) It can be used as a solvent for solution polymerization of polyisoprene rubber and cellulose ether. It can be used as a substitute for Freon as insulation materials in refrigerators and freezers as well as foaming agents for other hard PU foams, and chromatographic analysis standards. (2) Used as a standard substance for chromatographic analysis, solvents, engine fuels, azeotropic distillation agents. |
| Production method | Cyclopentane is a component of the petroleum ether in the 30-60 °C boiling point range with the content being generally 5%-10%. Apply atmospheric distillation; at a 60: 1 reflux ratio and carry out at an 8m height tower; first distill out the isopentane and n-pentane; continue fractionation to obtain a cyclopentane with purity being over 98%. Cyclopentane can also be obtained through cyclopentanone reduction or cyclopentadiene catalytic hydrogenation. |
| Hazards & Safety Information | Category Flammable liquids Toxicity grading: Low toxicity Acute toxicity: oral-rat LD50: 11400 mg/kg; oral-mouse LD50: 12800 mg/kg Hazardous property of explosives: being explosive upon mixed with air Flammability and dangerous situations: being flammable in case of fire, high temperature and oxidant with combustion releasing irritant smoke Storage characteristics: Storehouse: ventilated, low temperature and dry; Store it with oxidant separately Extinguishing agent: Dry powder, carbon dioxide, foam, 1211 extinguishing agent Occupational standard: TWA 1720 mg/m3; STEL 2150 mg/kg |
| Chemical Properties | colourless liquid with a petrol-like smell |
| Definition | ChEBI: A cycloalkane that consists of five carbons each bonded with two hydrogens above and below the plane. The parent of the class of cyclopentanes. |
| General Description | A clear colorless liquid with a petroleum-like odor. Flash point of -35°F. Less dense than water and insoluble in water. Vapors are heavier than air. |
| Air & Water Reactions | Highly flammable. Insoluble in water. |
| Reactivity Profile | CYCLOPENTANE is incompatible with strong oxidizing agents such as chlorine, bromine, fluorine. . |
| Health Hazard | Inhalation causes dizziness, nausea, and vomiting; concentrated vapor may cause unconsciousness and collapse. Vapor causes slight smarting of eyes. Contact with liquid causes irritation of eyes and may irritate skin if allowed to remain. Ingestion causes irritation of stomach. Aspiration produces severe lung irritation and rapidly developing pulmonary edema; central nervous system excitement followed by depression. |
| Fire Hazard | Behavior in Fire: Containers may explode. |
| Safety Profile | Mildly toxic by ingestion and inhalation. High concentrations have narcotic action. A very dangerous fire hazard when exposed to heat or flame; can react with oxidizers. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. |
| Purification Methods | Free it from cyclopentene by two passages through a column of carefully dried and degassed activated silica gel. It occurs in petroleum and is HIGHLY FLAMMABLE. [NMR: Christl Chem Ber 108 2781 1975, Whitesides et al. 41 2882 1976, Beilstein 5 III 10, 5 IV 4.] |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $100.00/200Kg/Drum |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2024-10-30 | |
| $10.50/1KG |
VIP4Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-09 | |
| $1100.00/1T |
VIP1Y
|
Yujiang Chemical (Shandong) Co.,Ltd.
|
2024-04-30 | |
| $100.00/1KG |
VIP2Y
|
Henan Fengda Chemical Co., Ltd
|
2023-12-26 | |
| $80.00/1KG |
Weijer International Trade (Hebei) Co., Ltd
|
2023-03-15 | ||
| $0.00/25KG |
VIP5Y
|
Hebei Mujin Biotechnology Co.,Ltd
|
2022-07-12 | |
| $0.00/1g |
Wuhan Godbullraw Chemical Co.,ltd
|
2022-05-11 | ||
| $0.00/1KG |
Wuhan Godbullraw Chemical Co.,ltd
|
2022-05-09 | ||
| $1.00/1KG |
XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD
|
2021-12-17 | ||
| $48.00/1KG |
Guangzhou Sunton Biotechnology Co., Ltd.
|
2021-04-14 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
楊俊青
sales@coreychem.com
sales@coreychem.com






 China
China