
CUMENE
| Price | $1 |
| Package | 1kg |
| Min. Order: | 1g |
| Supply Ability: | 100KG |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: CUMENE | CAS No.: 98-82-8 |
| Min. Order: 1g | Purity: 99% |
| Supply Ability: 100KG | Release date: 2019/07/06 |
| PRODUCT:: AD68 |
| CUMENE Basic information |
| Overview Production Uses Environmental Effects Safety Information |
| Product Name: | CUMENE |
| Synonyms: | (1-methylethyl)-benzen;(1-methylethyl)benzene (cumene);(Methylethyl)benzene;2-Fenilpropano;2-Fenyl-propaan;2-propylbenzene;Benzene, isopropyl-;benzene,isopropyl |
| CAS: | 98-82-8 |
| MF: | C9H12 |
| MW: | 120.19 |
| EINECS: | 202-704-5 |
| Product Categories: | Analytical Chemistry;Standard Solution of Volatile Organic Compounds for Water & Soil Analysis;Standard Solutions (VOC);Alpha Sort;Alphabetic;E-LAnalytical Standards;I;Volatiles/ Semivolatiles;C;CAlphabetic;CO - CZ;fine chemicals |
| Mol File: | 98-82-8.mol |
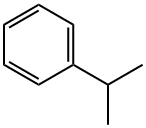 |
|
| CUMENE Chemical Properties |
| Melting point | −96 °C(lit.) |
| Boiling point | 152-154 °C(lit.) |
| density | 0.864 g/mL at 25 °C(lit.) |
| vapor density | 4.1 (vs air) |
| vapor pressure | 8 mm Hg ( 20 °C) |
| refractive index | n20/D 1.491(lit.) |
| Fp | 115 °F |
| storage temp. | 2-8°C |
| solubility | 0.05g/l |
| form | Liquid |
| color | Clear colorless |
| explosive limit | 0.8-6.0%(V) |
| Water Solubility | Soluble in alcohol, ether, acetone, benzene, carbon tetrachloride. Insoluble in water. |
| Merck | 14,2617 |
| BRN | 1236613 |
| Stability: | Stable, but may form peroxides in storage if in contact with the air. Test for the presence of peroxides before heating or distilling. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | RWGFKTVRMDUZSP-UHFFFAOYSA-N |
| CAS DataBase Reference | 98-82-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, (1-methylethyl)-(98-82-8) |
| EPA Substance Registry System | Benzene, (1-methylethyl)-(98-82-8) |
| Safety Information |
| Hazard Codes | Xn,N,T,F |
| Risk Statements | 10-37-51/53-65-39/23/24/25-23/24/25-11 |
| Safety Statements | 24-37-61-62-45-36/37-16 |
| RIDADR | UN 1918 3/PG 3 |
| WGK Germany | 1 |
| RTECS | GR8575000 |
| F | 10 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 29027000 |
| Hazardous Substances Data | 98-82-8(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 2.91 g/kg (Smyth) |
| MSDS Information |
| Provider | Language |
|---|---|
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| CUMENE Usage And Synthesis |
| Overview | Also known as 2-phenylpropane, Isopropylbenzene, or 1-methyl ethyl benzene, cumene is an aromatic hydrocarbon that is volatile, colorless liquid and has a smell similar to gasoline. Cumene is a natural component of crude oil, coal tar, and can be utilized as a blending component in gasoline. The compound is commonly found in Ceylan cinnamon and is a trace ingredient of ginger oil. |
| Production | Isopropylbenzene can be generated by alkylation or reacting benzene and propylene in the presence of an acid catalyst (1). Most of the modern plants that produce cumene use phosphoric acid as a catalyst, while some use a Friedel-Crafts reaction with aluminum trichloride (1). The reactions involved in the production of cumene from benzene and propylene are as follows: C3H6 + C6H6 = C6H5-C3H7 The catalytic process that is usually optimized at 25 atm. and 350oC is the best technology for the production of isopropylbenzene |
| Uses | Industrial Use Cumene is utilized in the production of phenol or its product, acetone that is widely used in the manufacture of plastics and petroleum products. It is also used in the production of methyl styrene and acetophenone as well as a thinner in lacquers, paints, and enamels. In addition, the compound is used as a solvent in the manufacture of iron, rubber, and steel as well as paper and pulp. Pharmaceutical Use Cumene hydroperoxide produces oxidative stress in the various steps of protein synthesis. Cumene hydroperoxide is used to probe isoenzyme specificity as well as region-and stereoselectivity. |
| Environmental Effects | Cumene vaporizes when released into the air where it is immediately reacted into other chemicals. However, in soil and water, bacteria degrade the chemical. Industrial emissions of the compound can lead to elevated concentrations in the atmosphere around the source. |
| Safety Information | Primarily, humans are exposed to cumene at industrial workplaces that use or produce the compound (2). However, reports have indicated low exposure concentrations during the production of cumene. On the other hand, succeeding reactions of the compound may occur in closed systems. Employers and workers must follow safe handling practices that are often found in the Manufacturer's Safety Data Sheet to enhance employee safety. Inhalation exposure to the chemical may cause drowsiness, dizziness, headaches, unconsciousness, and slight incoordination. It is an irritant when exposed to the skin and eye. As such, workers should wear protective clothing, gloves, respirators, and safety goggles. Furthermore, workplaces that cumene is being handled or produced should be well-ventilated to minimize the potential for employee exposure. |
| Chemical Properties | cumene is oxidized to its hydroperoxide, which is used to produce propene oxide. The alcohol produced is subsequently converted back to cumene over a copper-chromium oxide catalyst to be reused in the process. The advantage of this process is that cumene is easier to hydroperoxidate (more stable). |
| Air & Water Reactions | Flammable. Insoluble in water. |
| Reactivity Profile | Mixing CUMENE in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, nitric acid, oleum, NFPA 1991. |
| Sources and Potential Exposure | Cumene is a constituent of crude oil and finished fuels. It is released to the environment as a result of its production and processing from petroleum refining, the evaporation and combustion of petroleum products, and by the use of a variety of products containing cumene. The most probable route of human exposure is by the inhalation of contaminated air from the evaporation of petroleum products. Exposure may also occur through the consumption of contaminated food or water. |
| Health Hazard | Narcotic action with long-lasting effects; depressant to central nervous system. Acute (short-term) inhalation exposure to cumene may cause headaches, dizziness, drowsiness, slight incoordination, and unconsciousness in humans. Cumene has a potent central nervous system (CNS) depressant action characterized by a slow induction period and long duration of narcotic effects in animals. Cumene is a skin and eye irritant. No information is available on the chronic (long-term), reproductive, developmental, or carcinogenic effects of cumene in humans. Animal studies have reported increased liver, kidney, and adrenal weights from inhalation exposure to cumene. EPA has classified cumene as a Group D, not classifiable as to human carcinogenicity. |
| Fire Hazard | HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
| Safety Profile | Moderately toxic by ingestion. Mdly toxic by inhalation and skincontact. Human systemic effects by inhalation: an antipsychotic, unspecified changes in the sense of smell and respiratory system. An eye and skin irritant. Potential narcotic action. Central nervous system depressant. There is no apparent difference between the toxicity of natural cumene and that derived from petroleum. See also BENZENE and TOLUENE. Flammable liquid when exposed to heat or flame; can react with oxidizing materials. Violent reaction with HNO3, oleum, chlorosulfonic acid. To fight fKe, use foam, CO2, dry chemical. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $1.00/1kg |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2024-11-11 | |
| $100.00/1KG |
VIP2Y
|
Henan Fengda Chemical Co., Ltd
|
2023-12-26 | |
| $85.00/1kg |
VIP2Y
|
Zibo Hangyu Biotechnology Development Co., Ltd
|
2023-11-13 | |
| $100.00/1KG |
VIP5Y
|
Hebei Mujin Biotechnology Co.,Ltd
|
2023-09-05 | |
| $100.00/25kg |
Henan Bao Enluo International TradeCo.,LTD
|
2023-05-19 | ||
| $1.50/1g |
VIP3Y
|
Shaanxi Didu New Materials Co. Ltd
|
2022-05-18 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 | ||
| $4050.00/1T |
VIP4Y
|
Baoji Guokang Bio-Technology Co., Ltd.
|
2021-06-04 | |
| $10.00/1KG |
Wuhan Monad Medicine Tech Co.,LTD
|
2021-04-16 | ||
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-08 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
楊俊青
sales@coreychem.com
sales@coreychem.com






 China
China