
Tungsten hexafluoride
| Price | $1 |
| Package | 1kg |
| Min. Order: | 1 g |
| Supply Ability: | 100KG |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Tungsten hexafluoride | CAS No.: 7783-82-6 |
| EC-No.: 232-029-1 | Min. Order: 1 g |
| Purity: 99% | Supply Ability: 100KG |
| Release date: 2019/07/06 |
AD68
| Tungsten hexafluoride Basic information |
| Physical and Chemical Properties Hazardous characteristics Storage Instructions First-aid steps Category Flammability hazard characteristics Storage characteristics Extinguishing agent Occupational standards |
| Product Name: | Tungsten hexafluoride |
| Synonyms: | Tungstenfluoride;TUNGSTEN HEXAFLUORIDE;TUNGSTEN(VI) FLUORIDE;(oc-6-11)-tungstenfluoride(wf6;Hexafluorotungsten;Tungsten fluoride (WF6);Tungsten fluoride (WF6), (OC-6-11)-;WF6 |
| CAS: | 7783-82-6 |
| MF: | F6W |
| MW: | 297.83 |
| EINECS: | 232-029-1 |
| Product Categories: | Chemical Synthesis;Electronic Chemicals;Fluorides;Materials Science;Micro/NanoElectronics;Specialty Gases;Synthetic Reagents |
| Mol File: | 7783-82-6.mol |
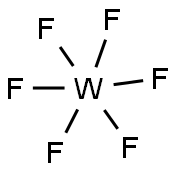 |
|
| Tungsten hexafluoride Chemical Properties |
| Melting point | 2.3 °C(lit.) |
| Boiling point | 17.5 °C(lit.) |
| density | d15liq 3.441 |
| form | colorless gas |
| Water Solubility | decomposes |
| Merck | 13,9885 |
| CAS DataBase Reference | 7783-82-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Tungsten hexafluoride(7783-82-6) |
| EPA Substance Registry System | Tungsten fluoride (WF6), (OC-6-11)-(7783-82-6) |
| Safety Information |
| Hazard Codes | T,C |
| Risk Statements | 23/24/25-34 |
| Safety Statements | 26-36/37/39-45 |
| RIDADR | UN 2196 2.3 |
| WGK Germany | 3 |
| RTECS | YO7720000 |
| Hazard Note | Toxic/Corrosive |
| HazardClass | 2.3 |
| MSDS Information |
| Provider | Language |
|---|---|
| Tungsten hexafluoride | English |
| SigmaAldrich | English |
| Tungsten hexafluoride Usage And Synthesis |
| Physical and Chemical Properties | Chemical formula is WF6. The molecular weight is 297.84. It is colorless gas or light yellow liquid. It has strong irritant. It is poisonous, its toxicity is similar with fluorine. When the temperature is very low, it is white solid. It is soluble in organic solvents and then generate a special color. When meet water, it can decompose. In the air, it can decompose with strong smoke by moisture, and generate yellow tungstate or tungsten trioxide. Chemical properties is lively. And almost all metals (except gold and platinum) can react with it. It is also corrosive for nickel, monel and stainless steel, but Ni and stainless steel have resistance for corrosion. When dry, it is less corrosive to glass, while in moisture, it is able to react quickly. It can react strong with gaseous ammonia. Ammonia or an alkali can absorb it . It can generate double salt with alkali metal fluoride. It is soluble in benzene and other organic solvents. [Density] 3.44×103kg/m3 (15℃, liquid) [Melting point] 2.5℃ (5.6 × 104 Pa) [Boiling point] 17.5℃ [Critical temperature] 171℃ [Critical pressure] 445.8kPa [Preparation method] Metal tungsten powder and elemental fluorine can react to synthesize it directly. [Application] (1) It is strong fluorinating agent, it can synthesize tungsten film in vapor deposition method. (2) it is used in the microelectronics industry for chemical vapor deposited tungsten silicide or tungsten, to produce a low resistance, a high melting point interconnects.  Figure 1 shows the molecular structure of tungsten hexafluoride. |
| Hazardous characteristics | When meet moisture, air or water, it will decompose and emit toxic and corrosive hydrogen fluoride fumes. It can cause very serious burns for skin, eyes, mucous membranes. When high concentrations expose, it can cause nausea, vomiting, abdominal pain, convulsions, kidney damage. The above information is edited by the chemicalbook of Wang Xiaodong. |
| Storage Instructions | It shoud be packed in special cylinders and stored in a cool, ventilated coffers. Avoid direct sunlight. Keep away from heat and sources of ignition. Prevent moisture. Metal, glass should be isolated storage. "poison gas" signs should be affixed. Aviation, railway transportation is prohibited. When other combustibles on fire around, dry powder, carbon dioxide can be used to extinguish. Water can be used to cool cylinder, and then close the valve quickly. |
| First-aid steps | It has eye irritation and can lead to blind, eyes should continue to be rinsed, go to the hospital fast for treatment. After corrosion, skin and mucous membranes is difficult to heal, it should be washed with water, and then coated with magnesium oxide ointment or glycerol dilute ammonia, and seek medical advice. |
| Category | Compressed gas and liquefied. |
| Flammability hazard characteristics | When meet water, it can decompose into toxic hydrogen fluoride gas and tungstate. |
| Storage characteristics | Treasury ventilation low-temperature drying. |
| Extinguishing agent | Water. |
| Occupational standards | TLV-TWA 5 mg (tungsten)/cubic meter; STEL 10 mg (tungsten)/cubic meter. |
| Chemical Properties | Colorless gas or light-yellow liquid. |
| Uses | In the electronics industry as a source of tungsten metal that connects the aluminum layers within semiconductor devices. |
| General Description | A toxic corrosive light yellow liquid or gas. Boiling point 67°F. Melting point 37°F. Noncombustible. Used in the manufacture of other chemicals and in the manufacture of electronics. |
| Air & Water Reactions | Decomposes in water giving hydrofluoric acid, another corrosive material. |
| Reactivity Profile | Tungsten hexafluoride emits very toxic and irritating fumes containing metallic tungsten and tungsten fluorides when heated to decomposition. Reacts violently with tetramethoxysilane [Jacob, E., Angew. Chem., 1982, 21, p. 143]. |
| Health Hazard | TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Vapors are extremely irritating and corrosive. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution. |
| Fire Hazard | Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $45.00/1000kg |
VIP1Y
|
Yujiang Chemical (Shandong) Co.,Ltd.
|
2025-01-16 | |
| $0.00/1kg |
VIP1Y
|
Wuhan Circle Star Chem-medical Technology Co.,Ltd
|
2025-01-08 | |
| $10.00/1KG |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2024-11-22 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
楊俊青
sales@coreychem.com
sales@coreychem.com




 China
China