(+)-Camptothecin NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-09-11 |
Product Details
| Product Name: (+)-Camptothecin | CAS No.: 7689-03-4 |
| EC-No.: 444-280-6 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/09/11 |
| CAS: | 7689-03-4 |
| MF: | C20H16N2O4 |
| MW: | 348.35 |
| EINECS: | 444-280-6 |
| Product Categories: | Antitumour;Signalling;Antitumors for Research and Experimental Use;Biochemistry;Quinoline Alkaloids;Natural Plant Extract;Chiral Reagents;Heterocycles;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Natural Anti-cancer Medical Materials and It's Derivatives;Antibiotics;Antibiotics A to;Alkaloids;Antineoplastic;Camptothecin series;Antibiotics A-FAntibiotics;Antibiotics by Application;Antineoplastic and Immunosuppressive AntibioticsAntibiotics;Inhibits an EnzymeAntibiotics;Interferes with DNA Synthesis;Mechanism of Action;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;inhibitor;natural product;7689-03-4 |
| Mol File: | 7689-03-4.mol |
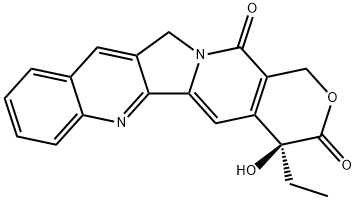 | |
| (+)-Camptothecin Chemical Properties |
| Melting point | 260 °C (dec.)(lit.) |
| alpha | D25 +31.3° (in chloroform-methanol, 8:2) |
| Boiling point | 482.73°C (rough estimate) |
| density | 1.3112 (rough estimate) |
| refractive index | 1.5700 (estimate) |
| storage temp. | 2-8°C |
| solubility | chloroform/methanol (4:1): 4 mg/mL |
| pka | pKa 10.83 (Uncertain) |
| form | solid |
| color | yellow |
| Water Solubility | insoluble |
| Merck | 14,1735 |
| BRN | 631069 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| InChIKey | VSJKWCGYPAHWDS-FQEVSTJZSA-N |
| LogP | 1.740 |
| CAS DataBase Reference | 7689-03-4(CAS DataBase Reference) |
| (+)-Camptothecin Usage And Synthesis |
| Description | Camptothecin is an alkaloid derived from Xi Shu (Camptotheca acuminata), which belongs to Nyssaceae. The traditional Chinese medicine Camptotheca acuminata (Xi Shu) has been collected in the Compilation of Chinese Herbal Medicine, Chinese Materia Medica, and Great Dictionary of Chinese Medicine. Camptotheca acuminata (Xi Shu) is widely distributed in the basin of Yangtze river and the southwestern provinces. The main medicinal parts of Camptotheca acuminata (Xi Shu) are root bark and fruit, which get rid of heat and toxic materials and eliminate the disease. |
| Description | DNA topoisomerases relax DNA torsional strain created during replication, transcription, recombination, repair, and chromosome condensation. The relaxation of DNA supercoiling by topoisomerase I at single- |
| Chemical Properties | light yellow crystal powde |
| Physical properties | Appearance: pale yellow needlelike crystal. Solubility: slightly soluble in ethanol and chloroform; poorly soluble in water; camptothecin fails to generate stable salt with acid, whereas it can produce sodium salt which is soluble in water by reacting with heated sodium hydroxide solution. Melting point: 264–267?°C. Camptothecin derivatives |
| History | In 1966, Wall M E et? al. from the United States isolated an alkaloid from Camptotheca acuminata and defined its chemical structure. The in?vitro anticancer tests revealed the anticancer activity of the tryptophan-terpene alkaloid, which is known as camptothecin and received widely concern. In 1975, Corey et?al. first opened the door for the chiral synthesis of camptothecin, but the reaction step was long and the yield rate was very low. It was not until 1997 that Ciufolini et?al. developed a new method for the synthesis of camptothecin by five steps, with a total yield rate up to 51%. The great breakthrough in the chemical synthesis of camptothecin has made its extensive application become a reality. Hydroxycamptothecin, as a camptothecin derivative with a hydroxyl group on the tenth carbon atom, is widely used for the treatment of various cancers. In 1969, researchers from Shanghai Institute of Materia Medica found that hydroxycamptothecin possessed potent anticancer activity and low toxicity. And this finding promoted the production and clinical application of hydroxycamptothecin, but its usage was interrupted for technology and quality. In the 1980s, hydroxycamptothecin was reproduced for clinical application with an improvement in producing technology, and hydroxycamptothecin got its approval number in 1986 for clinical usage in China. In the 1990s, the US Food and Drug Administration approved the clinical application of topotecan and irinotecan, which played a significant role in the prevention and treatment of cancers. |
| Uses | Antitumor alkaloid. Binds irreversible to the DNA-topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. A cytotoxic antitumor agent |
| Uses | antineoplastic |
| Uses | Antitumor agent;Topoisomerase I inhibitor |
| Uses | 10-hydroxycamptothecine precursor, topoisomerase inhibitor, binds irreversibly to DNA-topoisomerase I complex |
| Definition | ChEBI: A pyranoindolizinoquinoline that is pyrano[3',4':6,7]indolizino[1,2-b]quinoline which is substituted by oxo groups at positions 3 and 14, and by an ethyl group and a hydroxy group at position 4 (the S enantiomer). |
| Indications | It is mainly used in digestive tract tumors and has a good effect on gastric cancer, rectal cancer, and colon cancer. Besides, it can improve the surgical resection of advanced gastric cancer and also has some therapeutic effect on bladder cancer and lung adenocarcinoma. Moreover, camptothecin can be used for treatment of psoriasis, warts, acute and chronic leukemia, and hepatosplenomegaly caused by schistosomiasis. |
| Biological Activity | Cytotoxic plant alkaloid with antitumor properties; prototypic DNA topoisomerase I inhibitor. |
| Biochem/physiol Actions | (S)-(+)-Camptothecin binds irreversibly to the DNA-topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. The enzyme complex is ubiquinated and destroyed by the 26S proteasome, thus depleting cellular topoisomerase I. Blocks the cell cycle in S-phase at low does and induces apoptosis in a large number of normal and tumor cell lines by cell cycle-dependent and cell cycle-independent processes. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $10.00/1KG |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2024-11-28 | |
| $0.00/1g |
VIP1Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-10-11 | |
| $1.90/1KG |
VIP4Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-05 | |
| $1350.00/100g |
VIP2Y
|
R&D Scientific Inc.
|
2024-03-31 | |
| $0.00/1kg |
VIP2Y
|
Chongqing Zhihe Biopharmaceutical Co., Ltd.
|
2024-01-05 | |
| $1.00/1KG |
ANHUI SHENGZHIKAI BIOTECHNOLOGY CO.,LTD
|
2023-12-10 | ||
| $0.00/25kg |
VIP2Y
|
Chengdu ChenLv Herb Co.,Ltd
|
2023-08-04 | |
| $0.00/1KG |
VIP3Y
|
Changsha Staherb Natural Ingredients Co., Ltd.
|
2022-09-07 | |
| $1.10/1g |
VIP4Y
|
Dideu Industries Group Limited
|
2022-05-16 | |
| $3.00/1g |
Qiuxian Baitai New Material Co., LTD
|
2021-11-04 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 Japan
Japan