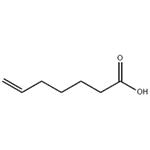
- 6-Heptenoic acid
-

- $0.00 / 200KG
-
2025-04-15
- CAS:1119-60-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- 6-Heptenoic acid
-
- $0.10 / 1KG
-
2024-07-14
- CAS:1119-60-4
- Min. Order: 1KG
- Purity: 98.0%
- Supply Ability: 10
- 6-HEPTENOIC ACID
-

- $0.00 / 25kg
-
2024-03-28
- CAS:1119-60-4
- Min. Order: 25kg
- Purity: 95%
- Supply Ability: Inquiry
|
| | 6-Heptenoic acid Basic information |
| | 6-Heptenoic acid Chemical Properties |
| Melting point | -6.5 °C (lit.) | | Boiling point | 222-224 °C (lit.) | | density | 0.946 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.439(lit.) | | Fp | >230 °F | | storage temp. | 2-8°C | | form | liquid | | pka | 4.75±0.10(Predicted) | | color | Colourless to light yellow | | Water Solubility | Miscible with water. | | BRN | 1747265 | | InChI | InChI=1S/C7H12O2/c1-2-3-4-5-6-7(8)9/h2H,1,3-6H2,(H,8,9) | | InChIKey | RWNJOXUVHRXHSD-UHFFFAOYSA-N | | SMILES | C(O)(=O)CCCCC=C | | LogP | 1.930 (est) |
| Hazard Codes | C | | Risk Statements | 34 | | Safety Statements | 26-36/37/39-45 | | RIDADR | UN 3265 8/PG 3 | | WGK Germany | 3 | | HazardClass | 8 | | PackingGroup | II | | HS Code | 2916199590 |
| | 6-Heptenoic acid Usage And Synthesis |
| Uses | 6-Heptenoic acid may be used in the preparation of 6-(iodomethyl)-hexanolide, via iodo-lactonization. | | Definition | ChEBI: 6-Heptenoic acid is a heptenoic acid with the double bond at position 6. | | Synthesis Reference(s) | Journal of the American Chemical Society, 107, p. 4230, 1985 DOI: 10.1021/ja00300a025
Tetrahedron, 51, p. 4991, 1995 DOI: 10.1016/0040-4020(95)98696-F
Tetrahedron Letters, 24, p. 4439, 1983 | | General Description | 6-Heptenoic acid is a straight-chain terminal alkenoic acid. Electrochemical oxidation of 6-heptenoic acid adsorbed on Pt(111) electrode surface has been reported. Preparation of 6-heptenoic acid has been reported. | | Synthesis | synthesis of 6-Heptenoic acid and higher homologs of this ω-unsaturated acid, a study of the pyrolysis of wheptano lactone was undertaken. The lactone was prepared by the oxidation of cycloheptanone under Baeyer-Villiger conditions. The pyrolysis of ω-unsaturated acid was effected by dropping the substance under a nitrogen atmosphere into a heated column packed with glass beads.
Synthesis of 6-Heptenoic Acid | | Precautions | Incompatible with strong oxidizing agents, strong acids and bases. |
| | 6-Heptenoic acid Preparation Products And Raw materials |
|