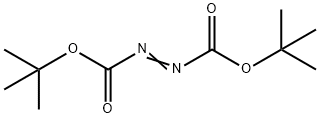
Di-tert-Butyl azodicarboxylate
|
|
Di-tert-Butyl azodicarboxylate ??
- ???
- 89-92 °C(lit.)
- ?? ?
- 287.1±9.0 °C(Predicted)
- ??
- 1.06±0.1 g/cm3(Predicted)
- ?? ??
- 2-8°C
- ???
- ?????(???? ??), ???(?? ???)
- ??? ??
- ?? ?? ??? ??
- ??
- ???
- ???
- ???
- ??
- Light Sensitive
- BRN
- 1911434
- InChI
- InChI=1S/C10H18N2O4/c1-9(2,3)15-7(13)11-12-8(14)16-10(4,5)6/h1-6H3
- InChIKey
- QKSQWQOAUQFORH-VAWYXSNFSA-N
- SMILES
- N(C(OC(C)(C)C)=O)=NC(OC(C)(C)C)=O
- CAS ??????
- 870-50-8(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi,F | ||
|---|---|---|---|
| ?? ???? ?? | 36/37/38-11 | ||
| ????? | 26-36-37/39-16 | ||
| ????(UN No.) | 1325 | ||
| WGK ?? | 3 | ||
| ?? ?? ?? | Irritant | ||
| ?? ?? | 3 | ||
| ???? | II | ||
| HS ?? | 29270000 |
Di-tert-Butyl azodicarboxylate C??? ??, ??, ??
??? ??
yellow crystals or crystalline powder??
Di-tert-butyl azodicarboxylate is a reagent used in the preparation of acyl hydrazinedicarboxylates. It is also used in the electrophilic amination of beta-keto esters catalyzed by an axially chiral guanidine. It serves as a precursor in an enantioselective synthesis of 3,6-dihyropyridazines employing organocatalysts such as L-proline or (S)-2-pyrrolidinyl tetrazole. It is also utilized in the asymmetric Friedel-Crafts amination through a chiral organocatalyst. Further, it acts as a reactant for preparation of hexapeptide key fragments through stereo selective selenocyclization/oxidative deselenylation reactions. In addition to this, it is employed as a starting material in the synthesis of pyrroloisoquinoline template through stereoselective N-acyliminium-mediated cyclization and enolate amination for preparation of peptidomimetic compounds and Barbier-type propargylation reactions.?? ??
Di-tert-butyl azodicarboxylate is a an acid labile reagent for Mitsunobu reactions allowing facile isolation of products and for the electrophilic amination and hydrazination of enolates and lithium alkyls.Purification Methods
The tert-butyl ester has the advantage over the ethyl ester (below) in being a solid and more acid labile. It crystallises from ligroin and is best purified by covering the dry solid (22g) with pet ether (b 30-60o, 35-40 mL) heating to boiling and adding ligroin (b 60-90o) until the solid dissolves. On cooling, large lemon yellow crystals of the ester separate (~ 20g), m 90.7-92o. Evaporation of the filtrate gives a further crop of crystals [Carpino & Crowley Org Synth 44 18 1964]. This reagent is useful in the Mitsunobu reaction [Mitsunobu Synthesis 1 1981, Gennari et al. J Am Chem Soc 108 6394 1986, Evans et al. J Am Chem Soc 108 6394 1986, Hughes Org React 42 335 1992, Dodge et al. Org Synth 73 110 1996, Hughes Org Prep Proc Int 28 127 1996, Ferguson & Marcelle J Am Chem Soc 128 4576 2006, see also DEAD and DIAD below].Di-tert-Butyl azodicarboxylate ?? ?? ? ???
???
?? ??
Di-tert-Butyl azodicarboxylate ?? ??
???( 388)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2930 | 58 |
| Ningxia LabsChem Co., Ltd. | 0952-9294929 +8613895679378 |
48472788@qq.com | China | 352 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12825 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 |
bess@weibangbio.com | China | 18145 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 3692 | 58 |
| Hebei Shengyang Water Conservancy Engineering Co., Ltd. | +8615373025980 |
clara@hbshengyang.com | China | 896 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29685 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 19881 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21632 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1803 | 55 |