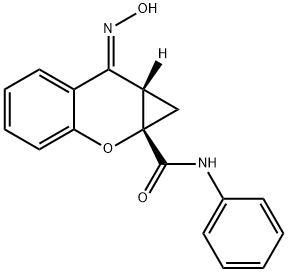
N-PHENYL-7-(HYDROXYIMINO)CYCLOPROPA[B]CHROMEN-1A-CARBOXAMIDE
N-PHENYL-7-(HYDROXYIMINO)CYCLOPROPA[B]CHROMEN-1A-CARBOXAMIDE ??? ???
?? ??:
1161205-27-1
???:
N-PHENYL-7-(HYDROXYIMINO)CYCLOPROPA[B]CHROMEN-1A-CARBOXAMIDE
???(??):
PHCCC;N-PHENYL-7-(HYDROXYIMINO)CYCLOPROPA[B]CHROMEN-1A-CARBOXAMIDE;(1aS,7E,7aS)-7-hydroxyimino-N-phenyl-1,7a-dihydrocyclopropa[b]chromene-1a-carboxamide;(1aS,7E,7aS)-7,7a-Dihydro-7-(hydroxyimino)-N-phenylbenzo[b]cyclopropa[e]pyran-1a(1H)-carboxamide;Benzo[b]cyclopropa[e]pyran-1a(1H)-carboxamide, 7,7a-dihydro-7-(hydroxyimino)-N-phenyl-, (1aS,7E,7aS)-
CBNumber:
CB0450874
???:
C17H14N2O3
??? ??:
294.3
MOL ??:
Mol file
N-PHENYL-7-(HYDROXYIMINO)CYCLOPROPA[B]CHROMEN-1A-CARBOXAMIDE ??
?? ?
579.1±50.0 °C(Predicted)
??
1.41±0.1 g/cm3(Predicted)
?? ??
Store at RT
?? ?? (pKa)
10.79±0.40(Predicted)
??
N-PHENYL-7-(HYDROXYIMINO)CYCLOPROPA[B]CHROMEN-1A-CARBOXAMIDE C??? ??, ??, ??
???? ??
Group I metabotropic glutamate receptor antagonist (IC 50 ~ 3 μ M); 67 times more potent than (S)-4-carboxyphenylglycine ((S)-4-Carboxyphenylglycine). Also a positive allosteric modulator for mGlu 4a ; potentiates L-AP4-mediated inhibition of striatopallidal synaptic transmission in vitro . Displays antiParkinsonian effects in rats in vivo .
N-PHENYL-7-(HYDROXYIMINO)CYCLOPROPA[B]CHROMEN-1A-CARBOXAMIDE ?? ?? ? ???
???
?? ??
N-PHENYL-7-(HYDROXYIMINO)CYCLOPROPA[B]CHROMEN-1A-CARBOXAMIDE ?? ??
???( 40)?? ??
China 34
United Kingdom 5
United States 1
Global 40