フェニルヒドロキシアミン 化學(xué)特性,用途語,生産方法
化學(xué)的特性
tan powder or crystals
使用
Manufacture of cupferron.
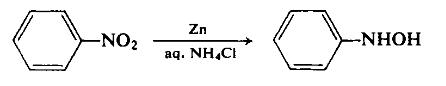
製造方法
To a dispersion of 180 gm (2.75 gm-atom) of zinc dust in 500 ml of 50% aqueous ethanol, with vigorous stirring, is added 130 ml (156 gm, 1.27 mole) of nitrobenzene. The reaction is initiated by the dropwise addition of an aqueous ammonium chloride solution and can thereafter be maintained at a controllable rate by the cautious addition of the remaining ammonium chloride solution. The reaction temperature rises during this reduction step. Once reflux has subsided, the basic zinc salts are filtered off using a sintered glass funnel. The light green filtrate is cooled in an ice-salt mixture to precipitate the product. The yield is 100 gm (72.2%), m.p. 81°C.
The reduction of 2-methyl-2-nitropropane is carried out in a similar manner at 10-20°C to afford a 68% yield of N-t-butylhydroxylamine (m.p. 60-62°C).
一般的な説明
Tan to brown crystals.
空気と水の反応
Soluble in hot water.
反応プロフィール
N-Phenylhydroxylamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
火災(zāi)危険
Flash point data for N-Phenylhydroxylamine are not available but N-Phenylhydroxylamine is probably nonflammable.
安全性プロファイル
Poison by ingestion and subcutaneous routes. Human systemic effects by skin contact: primary irritation. Preparative hazard. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
純化方法
Impure base deteriorates rapidly. Crystallise it from H2O, *C6H6 or *C6H6/pet ether (40-60o). The picrate has m 186o (from EtOH), and the benzenesulfonate salt has m 70o (dec )(EtOH/*C6H6). [Beilstein 15 H 2, 15 I 3, 15, II 4, 15 III 5. 15 IV 4.]
フェニルヒドロキシアミン 上流と下流の製品情報(bào)
原材料
準(zhǔn)備製品