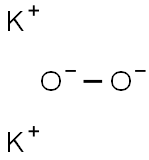potassium peroxide
- CAS No.
- 17014-71-0
- Chemical Name:
- potassium peroxide
- Synonyms
- Peroxydipotassium;POTASSIUM PEROXIDE;Dipotassio peroxide;DUPSQGGNCHNYTQ-UHFFFAOYSA-M
- CBNumber:
- CB22131115
- Molecular Formula:
- K2O2
- Molecular Weight:
- 110.2
- MDL Number:
- MOL File:
- 17014-71-0.mol
| Melting point | 490°C |
|---|---|
| Density | >1 g/cm3 |
| solubility | reacts with H2O |
| form | yellow amorphous solid |
| color | yellow amorphous solid |
| Water Solubility | decomposed by H2O [HAW93] |
| CAS DataBase Reference | 17014-71-0 |
| FDA UNII | ZHB4ZOE9PU |
| EPA Substance Registry System | Dipotassium peroxide (17014-71-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H272-H315 | |||||||||
| Precautionary statements | P210-P220-P221P280-P370+P378-P501-P264-P280-P302+P352-P321-P332+P313-P362 | |||||||||
| RIDADR | UN 1491 5.1/ PGI | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | I | |||||||||
| Hazardous Substances Data | 17014-71-0(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
potassium peroxide Chemical Properties,Uses,Production
Preparation
Potassium peroxides have been prepared by carefully controlled oxidation of the metals with the exact amount of air or nitric oxide, but it is difficult to prepare the pure peroxides in this manner because of the ease with which the peroxides are oxidized to the superoxide. They can also be prepared by careful oxidation of liquid ammonia solutions of the metals, using the stoichiometric quantity of oxygen for peroxide formation. They can also be prepared by the thermal decomposition of the superoxides in vacuum; for example, potassium superoxide decomposes at about 400°C.
Chemical Properties
yellow amorphous mass; decomposes in water, evolving oxygen; oxidizing agent; used for bleaching, in oxygen generating gas masks [HAW93]
Uses
Oxidizing agent, bleaching agent, oxygen- generating gas masks.
General Description
A yellow granular solid. Mixtures of potassium peroxide and combustible material readily ignited by friction, heat or contact with moisture. Prolonged exposure to fire or heat may cause vigorous decomposition of the material and rupture of the container. Used as a bleach.
Air & Water Reactions
Reacts exothermically with water (or moisture in the air) to give oxygen and a caustic solution, potassium hydroxide [NFPA 491M] .
Reactivity Profile
potassium peroxide is a strong oxidizing agent. Reacts readily with reducing agents, including most organic compounds, to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). A quantity left on a piece of paper in the open air usually starts a fire in the paper spontaneously in a few minutes [Freeman].
Hazard
Dangerous fire and explosion risk in con- tact with organic materials, strong oxidizing agent. Irritant to skin and tissue.
Health Hazard
Inhalation causes respiratory irritation. Ingestion causes severe burns of mouth and stomach. Contact with eyes or skin causes irritation and caustic burns.
Fire Hazard
Behavior in Fire: Increases intensity of fire and can start fires when in contact with organic combustibles
Safety Profile
Dangerous fire hazard by spontaneous chemical reaction. It is a very powerful oxidzer. Fires of this material should be handled like sodurn peroxide fires. Moderate explosion hazard by spontaneous chemical reaction. Explodes on contact with water, forming H2O2 and KOH. Violent reactions with air, Sb, As, O2 , K. Vigorous reaction on contact with reducing materials. On contact with acid or acid fumes, it can emit toxic fumes. Incompatible with carbon, diselenium dichloride, ethanol, hydrocarbons, metals. When heated to decomposition it emits toxic fumes of K2O . See also PEROXIDES, E INORGANIC.