70181-03-2
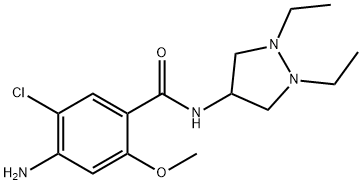 70181-03-2 結(jié)構(gòu)式
70181-03-2 結(jié)構(gòu)式
基本信息
Dazopride
4-amino-5-chloro-N-(1,2-diethylpyrazolidin-4-yl)-2-methoxybenzamide
4-Amino-5-chloro-N-(1,2-diethyl-4-pyrazolidinyl)-2-methoxybenzamide
Benzamide, 4-amino-5-chloro-N-(1,2-diethyl-4-pyrazolidinyl)-2-methoxy-
常見問題列表
Dazopride (0.3 mg/kg) produces significant enhancement of gastric evacuation and is approximately six times more potent than metoclopramide in gastric evacuation assay. Dazopride (0.3-10.0 mg/kg, i.v.) produces a dose-related increase in antral motility primarily by increasing the amplitude of antral contractions in three conscious dogs. Dazopride significantly reduces the emetic frequency from that of the control group. Dazopride (5 mg/kg, i.p.) antagonises the tetralin-induced emesis in all animals, but fails to antagonise the response at 0.25-2.5 mg/kg. Dazopride fails to modify cisplatin-induced emesis at 0.1 mg/kg (i.v,) although a larger dose of 1.0 mg/kg abolishes or attenuates the response and 5.0 mg/kg of dazopride antagonises the development ofemesis in all animals.