286930-02-7
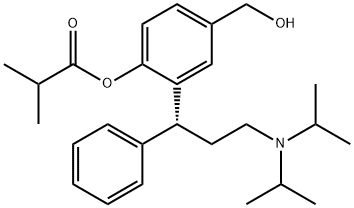 286930-02-7 結(jié)構(gòu)式
286930-02-7 結(jié)構(gòu)式
基本信息
中文別名
弗斯特羅定富馬酸弗斯特羅定雜質(zhì)
英文別名
PF-00695838Fesoterodine
Unii-621G617227
(R) Fesoterodine
2-Methylpropionic acid 2-[3-(N,N-diisopropylamino)-1(R)-phen...
(R)-2-(3-(diisopropylamino)-1-phenylpropyl)-4-(hydroxymethyl)phenyl isobutyrate
2-Methylpropanoic acid 2-[(1R)-3-[bis(1-methylethyl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl ester
Propanoic Acid 2-Methyl- 2-[(1R)-3-[bis(1-methylethyl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl Ester
Desfesoterodine,Fesoterodine,OAB,muscarinic,overactive,Inhibitor,SPM7605,mAChR,orally,5-HMT,antimuscarinic,bladder,Muscarinic acetylcholine receptor,inhibit,receptor
物理化學(xué)性質(zhì)
密度1.043
儲存條件Store at -20°C,unstable in solution, ready to use.
溶解度Ethanol: 100 mg/mL (242.97 mM)
形態(tài)Viscous Liquid
顏色Colorless to light yellow
常見問題列表
概述
弗斯特羅定(fesoterodine,商品名ToviazTM)是一種新型M受體阻滯劑,在體內(nèi)迅速代謝成活性代謝產(chǎn)物而發(fā)揮作用。用途
弗斯特羅定于2008年10月31日獲得美國FDA批準(zhǔn)上市,用于治療膀胱過度活動癥(overactivebladder,OAB)。生物活性
Fesoterodine 是一種口服有效,非亞型選擇性的,競爭性毒蕈堿受體 (mAChR) 拮抗劑,對 M1,M2,M3,M4,M5 受體的 pKi 值分別為 8.0,7.7,7.4,7.3,7.5。Fesoterodine 用于膀胱過度活動癥 (OAB)。靶點(diǎn)
pKi: 8.0 (M1), 7.7 (M2), 7.4 (M3), 7.3 (M4) and 7.5 (M5)
體外研究
Fesoterodine decreases micturition frequency, urgency severity and urgency incontinence episodes and increases the volume voided with each micturition.
After oral administration, Fesoterodine is rapidly and extensively hydrolysed in plasma by nonspecific esterases to Desfesoterodine (5-hydroxymethyl tolterodine; SPM 7605; HY-76569; an active metabolite of Fesoterodine).
體內(nèi)研究
Fesoterodine (0.01-1 mg/kg; IV) reduces the micturition pressure and increases bladder capacity and ICIs (intercontraction intervas) at the lowest dose tested of 0.01 mg/kg.
| Animal Model: | Bladders from female Sprague-Dawley rats (225-275 g) |
| Dosage: | 0.01, 0.1 and 1 mg/kg |
| Administration: | IV |
| Result: | Reduced the micturition pressure and increased bladder capacity and ICIs at the lowest dose tested of 0.01 mg/kg. |
