1802637-39-3
中文名稱
CS-2826
英文名稱
BB-Cl-Amidine
CAS
1802637-39-3
分子式
C26H26ClN5O
分子量
459.97
MOL 文件
1802637-39-3.mol
更新日期
2024/06/25 17:15:55
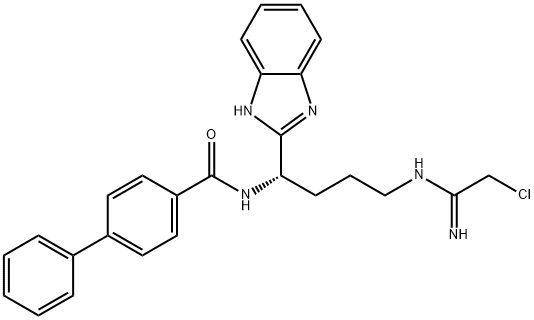 1802637-39-3 結(jié)構(gòu)式
1802637-39-3 結(jié)構(gòu)式
基本信息
中文別名
(S)-N-(1-(1H-苯并[D]咪唑-2-基)-4-(2-氯乙酰亞胺基)丁基)-[1,1'-聯(lián)苯基]-4-甲酰胺 英文別名
CS-2826BB-Cl-Amidine
N-{(1S)-1-(1H-Benzimidazol-2-yl)-4-[(2-chloroethanimidoyl)amino]butyl}-4-biphenylcarboxamide
[1,1'-Biphenyl]-4-carboxamide, N-[(1S)-1-(1H-benzimidazol-2-yl)-4-[(2-chloro-1-iminoethyl)amino]butyl]-
物理化學(xué)性質(zhì)
儲(chǔ)存條件Inert atmosphere,Store in freezer, under -20°C
溶解度DMSO:76.33(Max Conc. mg/mL);165.94(Max Conc. mM)
DMF:20.0(Max Conc. mg/mL);43.48(Max Conc. mM)
Water:70.0(Max Conc. mg/mL);152.18(Max Conc. mM)
Ethanol:54.5(Max Conc. mg/mL);118.48(Max Conc. mM)
Ethanol:PBS (pH 7.2) (1:1):0.5(Max Conc. mg/mL);1.09(Max Conc. mM)
DMF:20.0(Max Conc. mg/mL);43.48(Max Conc. mM)
Water:70.0(Max Conc. mg/mL);152.18(Max Conc. mM)
Ethanol:54.5(Max Conc. mg/mL);118.48(Max Conc. mM)
Ethanol:PBS (pH 7.2) (1:1):0.5(Max Conc. mg/mL);1.09(Max Conc. mM)
形態(tài)A crystalline solid
顏色white to beige
CS-2826價(jià)格(試劑級(jí))
| 報(bào)價(jià)日期 | 產(chǎn)品編號(hào) | 產(chǎn)品名稱 | CAS號(hào) | 包裝 | 價(jià)格 |
| 2025/02/08 | HY-111347 | CS-2826 BB-Cl-Amidine | 1802637-39-3 | 5 mg | 950元 |
| 2025/02/08 | HY-111347 | CS-2826 BB-Cl-Amidine | 1802637-39-3 | 10 mg | 1700元 |
| 2025/02/08 | HY-111347 | CS-2826 BB-Cl-Amidine | 1802637-39-3 | 25 mg | 3600元 |
常見問題列表
生物活性
BB-Cl-Amidine 是肽基精氨酸脫胺酶 (PAD) 的抑制劑。靶點(diǎn)
PAD.
體內(nèi)研究
Treatment with BB-Cl-amidine subtly reduces splenomegaly in MRL/lpr mice, while there is a trend towards increased circulating levels of anti-NET antibodies with PAD inhibitor treatment. However, neither PAD inhibitor affected body weight or total IgG levels. Indeed, treatment with both Cl-amidine and BB-Cl-amidine significantly improves endothelium-dependent vasorelaxation. The BB-Cl-amidine group also shows a strong trend towards downregulation of IRGs. Treatment with either Cl-amidine or BB-Cl-amidine significantly improves muzzle alopecia, in many cases preventing it entirely.
| Animal Model: | MRL/lpr mice. |
| Dosage: | 1 mg/kg. |
| Administration: | Subcutaneous injection daily from 8 to 14 weeks of age. |
| Result: | Significantly improved endothelium-dependent vasorelaxation and showed a strong trend towards downregulation of IRGs. |
