132418-35-0
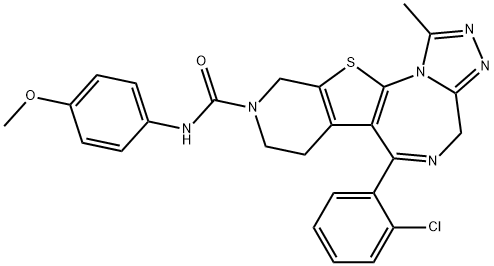 132418-35-0 結(jié)構(gòu)式
132418-35-0 結(jié)構(gòu)式
基本信息
4H-Pyrido[4',3':4,5]thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine-9(8H)-carboxamide, 6-(2-chlorophenyl)-7,10-dihydro-N-(4-methoxyphenyl)-1-methyl-
常見問題列表
PAF
Animals are separated into six groups: U 4 , controls; S, sham operated animals undergoing laparotomy; I 4 and I 9 , ligation of the mesenteric vessels in the last ileal loop; IT 4 and IT 9 , same procedure together with treatment with Setipafant (50 mg/kg) orally before and after surgery and intraperitoneally during surgery. Animals are killed at day 4 in groups U 4 , S, I 4 and IT 4 and at day 9 in groups I 9 and IT 9 , with histological studies and mediator measurements taken. Macroscopic and histological lesions of intestinal wall in groups I 4 , I 9 , IT 4 and IT 9 are similar to those of human neonatal necrotizing enterocolitis and do not vary according to the absence or the presence of Setipafant (BN 50727) treatment. Peritoneal bands are significantly reduced in treated groups IT 4 and IT 9 as compared with untreated ones I 4 and I 9 . Mucosal PAF levels in the terminal ileum are higher in group I 4 than in groups U 4 or I 9 . In the upper loop, mucosal PAF levels are comparable in all groups. An increase in stool PAF levels is observed only in group I 9 , whereas values comparable to those observed in controls are detected in other groups. Pretreatment of the animals with one or other of the structurally unrelated PAF receptor antagonists, BN 52021 (10 mg/kg, i.p.) or BN 50727 (1 mg/kg, i.p.) significantly reduces Dexamethasone-induced gastric damage. In these animals neither petechiae nor erosions are observed.
