130477-52-0
中文名稱
130477-52-0
英文名稱
L-655,708
CAS
130477-52-0
分子式
C18H19N3O4
分子量
341.36
MOL 文件
130477-52-0.mol
更新日期
2025/02/06 17:30:36
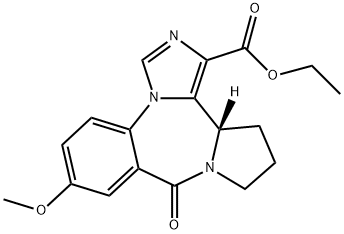 130477-52-0 結(jié)構(gòu)式
130477-52-0 結(jié)構(gòu)式
基本信息
中文別名
(S)-7-甲氧基-9-氧代-11,12,13,13A-四氫-9H-9H-咪唑并[1,5-A]吡咯并[2,1-C][1,4]苯二氮-1-羧酸乙酯 英文別名
MSDL-655,708
ETHYL (S)-11,12,13,13A-TETRAHYDRO-7-METHOXY-9-OXO-9H-IMIDAZO[1,5-A]PYRROLO[2,1-C][1,4]BENZODIAZEPINE-1-CARBOXYLATE
Ethyl (S)-11,12,13,13a-Tetrahydro-7-methoxy-9-oxo-9H-imidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylate
11,12,13,13a-Tetrahydro-7-methoxy-9-oxo-9H-imidazo[1.5-a]pyrrolo[2.1-c][1.4]benzodiazepine-1-carboxylicacidethylester
(S)-ethyl 7-methoxy-9-oxo-11,12,13,13a- tetrahydro-9H-benzo[e]imidazo[5,1-c]pyrrolo[1,2-a][1,4]diazepine-1-carboxylate
11,12,13,13A-TETRAHYDRO-7-METHOXY-9-OXO-9H-IMIDAZO[1,5-A]PYRROLO[2,1-C][1,4]BENZODIAZEPINE-1-CARBOXYLIC ACID, ETHYL ESTER
9H-IMidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylic acid, 11,12,13,13a-tetrahydro-7-Methoxy-9-oxo-, ethyl ester, (13aS)-
9H-IMidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylic acid, 11,12,13,13a-tetrahydro-7-Methoxy-9-oxo-, ethyl ester, (13aS)- (011-14426_500Mg)
所屬類別
生物化工:生化試劑物理化學(xué)性質(zhì)
熔點(diǎn)175-176 °C
沸點(diǎn)584.4±50.0 °C(Predicted)
密度1.42±0.1 g/cm3(Predicted)
儲(chǔ)存條件Desiccate at +4°C
溶解度DMSO: 6 mg/mL
溶解度二甲基亞砜:6 mg/毫升
酸度系數(shù)(pKa)1.49±0.20(Predicted)
形態(tài)powder
顏色White to yellow
化合物L(fēng)-655708價(jià)格(試劑級(jí))
| 報(bào)價(jià)日期 | 產(chǎn)品編號(hào) | 產(chǎn)品名稱 | CAS號(hào) | 包裝 | 價(jià)格 |
| 2025/02/08 | HY-14426 | 130477-52-0 L-655708 | 130477-52-0 | 5mg | 880元 |
| 2025/02/08 | HY-14426 | 130477-52-0 L-655708 | 130477-52-0 | 10mM * 1mLin DMSO | 968元 |
| 2025/02/08 | HY-14426 | 130477-52-0 L-655708 | 130477-52-0 | 10mg | 1550元 |
常見問題列表
生物活性
L-655708 是有效的, 選擇性 GABAA 受體反向激動(dòng)劑 (Ki=0.45 nM)。體外研究
L655708 is a potent, selective inverse agonist for the benzodiazepine site of GABAA receptors containing the α5 subunit (Ki = 0.45 nM). Displays 50-100-fold selectivity over GABAA receptors containing α1, α2, α3 orα6 subunits in combination with β3 and γ2. Enhances LTP in a mouse hippocampal slice model and increases spatial learning, without displaying proconvulsant activity.
體內(nèi)研究
L-655708 at 0.7 mg/kg, administered intraperitoneally, would result in 60-70% occupancy of α5 GABAA receptors with limited binding to α1, α2, and α3 subunit-containing GABAA receptors and no significant off-target behavioral effects, such as sedation and motor impairment.