| Identification | Back Directory | [Name]
Imidazole-1-sulfonyl azide hydrochloride | [CAS]
952234-36-5 | [Synonyms]
Imidazole-1-sulfonyl azide HCl
Imidazole-1-sulfonyl azide, HCl salt
Imidazole-1-sulfonyl azide hydrochloride
1H-Imidazole-1-sulphonylazidehydrochloride
1H-Imidazole-1-sulfonyl azide hydrochloride
N-diazoimidazole-1-sulfonamide hydrochloride
1H-Imidazole-1-sulfonyl Azide Hydrochloride Salt | [Molecular Formula]
C3H3N5O2S.HCl | [MDL Number]
MFCD19705431 | [MOL File]
952234-36-5.mol | [Molecular Weight]
210 |
| Chemical Properties | Back Directory | [Melting point ]
102-104℃ | [storage temp. ]
2-8°C | [solubility ]
Methanol (Slightly), Water (Slightly) | [form ]
Solid | [color ]
White to Off-White | [Stability:]
Hygroscopic, forms hydrazoic acid on prolonged storage and on contact with water |
| Hazard Information | Back Directory | [Uses]
1H-Imidazole-1-sulfonyl azide hydrochloride(952234-36-5) is an azide salt, which can be used as an intermediate in organic synthesis and material chemistry, and is commonly used as a diazotropic transfer reagent in the field of chemical synthesis. It is also used in the synthesis of bioactive molecules and organic functional materials.
| [reaction suitability]
reaction type: click chemistry | [Synthesis]
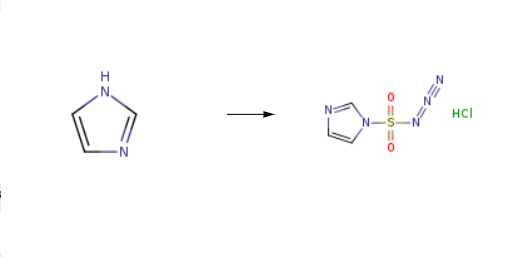
1H-Imidazole-1-sulfonyl azide hydrochloride(952234-36-5) was synthesised by a series of chemical reactions using 1H-imidazole as raw material, and the specific reflective steps are as follows:
Sulfurylchloride (16.2 mL, 0.2 mol) was added dropwise to an ice-cooled suspension ofNaN3 (13.0 g, 0.2 mol) in anhydrous acetonitrile (200 mL) and themixture was stirred overnight. Imidazole (27.2 g, 0.4 mol) was addedportionwise to the ice-cooled mixture and the slurry was stirred for 5 h. Themixture was diluted by EA (400 mL) and 400 mL of water was added. The organiclayer was washed by water (2x400 mL), followed by saturated NaHCO3solution (2x400 mL) and brine (400 mL). The organic layer was dried over Na2SO4.Asolution of HCl/EtOH, obtained by dropwise addition of AcCl (21.3 mL, 0.3 mol)to ice-cooled dry ethanol (75 mL) was added dropwise to the filtrate withstirring, which was dried to get the white solid (36 g, 96% yield), which waskept desiccator under N2 atmosphere.LC-MS(ESI): [M+1]+ = 173.80, tR = 2.73 min.1H NMR (400 MHz, D2O) δ 9.43 (s, 1H), 8.00 (s, 1H), 7.60 (s, 1H).13C NMR (101 MHz, D2O) δ 137.66, 123.01, 120.20.
|
|
|