| Identification | Back Directory | [Name]
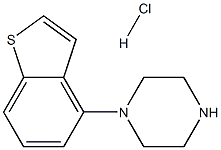
1-(1-Benzothiophen-4-yl)piperazine hydrochloride | [CAS]
913614-18-3 | [Synonyms]
1-benzo[b]thien-4-yl-
Brexpiprazole intermediate
Brexpiprazole Intermediate 2
Brexpiprazole Impurity 32 HCl
1-benzo[b]thien-4-yl-, hydrochloride
1-Benzo[b]thien-4-yl-piperazinel HCl
4-piperazinyl benzothiophene hydrochloride
1-(4-Benzothienyl)piperazine Hydrochloride
PIPERAZINE, 1-BENZO[B]THIEN-4-YL,HYDROCLORIDE
1-Benzo[b] thien-4-yl-piperzine hydrochloride
1-Benzo[b]thien-4-yl-piperazine hydrochloride
Piperazine, 1-benzo[b]thien-4-yl-, hydrochloride
1-Benzo[b]thien-4-ylpiperazine Monohydrochloride
1-(1-benzothiophen-4-yl)piperazine hydrochloride
1-(benzo[b]thiophen-4-yl)piperazine hydrochloride
1-benzo[b]thiophene-4-yl-piperazine hydrochloride
1-(1-benzo[b]thien-4-yl )piperazine hydrochloride
1-benzo[b]thien-4-yl-Piperazine hydrochloride (1:1)
1-(1-Benzothiophene-4-Yl)Piperazine Hydrochloroide -
1-(1-benzothiophene-4-yl)piperazine monohydrochloride
7-(4-chlorobutoxy)-1-(4-chlorobutyl)quinolin-2(1H)-one
Piperazine, 1-benzo[b]thien-4-yl-, hydrochloride (1:1)
Brexpiprazole Impurity 11
1-(benzo[b]thiophen-4-yl)piperazine hydrochloride | [EINECS(EC#)]
812-454-4 | [Molecular Formula]
C12H15ClN2S | [MDL Number]
MFCD26743820 | [MOL File]
913614-18-3.mol | [Molecular Weight]
254.779 |
| Chemical Properties | Back Directory | [Melting point ]
>250°C (dec.) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
DMSO (Slightly, Heated), Methanol (Very Slightly) | [form ]
Solid | [color ]
White |
| Hazard Information | Back Directory | [Uses]
4-Piperazinylbenzothiophene hydrochloride is the raw material for preparing piperazine-substituted benzothiophene, which can be used for the treatment and prevention of mental diseases including central nervous system diseases. | [Synthesis]
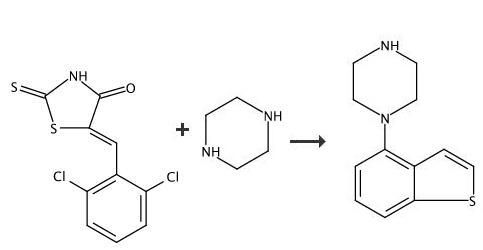
Synthesis of 4-(1-piperazinyl)benzo[b]thiophene hydrochloride 4-Chlorobenzo[b]thiophene (5.00 g), piperazine (5.11 g), palladium acetate (II) (2.7 mg), tri-tert-butylphosphonium tetraphenylborate (6.2 mg), sodium tert-butoxide (8.548 g), and xylene (70 ml) were stirred at 120 to 130°C for 5 hours. After the reaction mixture was cooled to room temperature, water was added thereto, and the layers were separated. The xylene layer was washed with water, and then with saline. After addition of activated carbon, the mixture was stirred at room temperature for 30 minutes. After filtration of the mixture, concentrated hydrochloric acid was added to the filtrate, and the resulting mixture was stirred at room temperature for 30 minutes. The precipitated crystals were collected by filtration and dried to obtain compound. Yield: 6.94 g. 1H-NMR (DMSO-d6) δ ppm; 3.30 (4H, br.s), 3.61 (4H, br.s), 6.97 (1H, d, J = 7.8 Hz), 7.32 (1H, br. dd, J = 8.4, 7.8 Hz), 7.53 (1H, d, J = 5.6 Hz), 7.70 (1H, d, J = 8.4 Hz), 7.76 (1H, d, J = 5.6 Hz), 9.37 (1H, br.s). |
|
|