| Identification | Back Directory | [Name]
Vasopressin | [CAS]
9034-50-8 | [Synonyms]
Vasopressin
Vasopressin acetate salt
TIANFU-CHEM Vasopressin | [Molecular Formula]
C43H67N15O12S2 | [MDL Number]
MFCD03839092 | [Molecular Weight]
1050.22 |
| Questions And Answer | Back Directory | [Discovery]
Vasopressin is a mammalian VP family peptide with vasopressor and antidiuretic actions. Disorders of VP and V2aR
cause central and nephrogenic diabetes insipidus,
respectively. Vasopressor and the antidiuretic effects of VP were
first reported in 1895 and 1913, respectively. Arginine
vasopressin (AVP) was isolated in 1951, and chemically
synthesized in 1955. | [Properties]
Mr 1084 (AVP), 1056 (LVP), 1068 (phenypressin); pI,
10.9 (AVP). The peptides are freely soluble in water.
AVP solution in water at >10-4M is stable for more than
a year at -20°C, with increased stability under acidic conditions using acetic acid. | [Structure]
The N-terminal six aa residues of the nonapeptides are
flanked by two cysteine residues (positions 1 and 6) forming an intramolecular ring while the C-terminal extension
with arginine or lysine residue confers a basic peptide
property. The C-terminus is amidated. VPs are found
only in mammals. Three peptides have been found . Most
mammals, including humans, have AVP while LysineVP (LVP) and phenypressin were discovered from pigs
and marsupials, respectively.
 | [Gene, mRNA, and precursor]
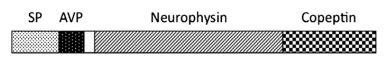
The human preproAVP gene located on the short arm of chromosome 20 (20p13) consists of three exons and two introns.2 The oxytocin (OT) gene is located in the vicinity of the AVP gene in a tail-to-tail configuration, and they are transcribed in opposite directions. Human AVP mRNA has 595 bp encoding a signal peptide, a mature AVP with processing and amidation sites (GlyLys-Arg), a neurophysin, and a glycoprotein (copeptin) . Processing of the prohormone occurs in the acidic pH of large dense core vesicles. The neurophysin noncovalently binds with the mature peptide in the vesicles and functions as an intravesicular chaperone and carrier protein while the function of copeptin is still unknown. The AVP gene has glucocorticoid and cAMP responsive elements, and AP-1/2 regulatory elements in the promoter region. A downstream region of the AVP gene is also important as an enhancer.
| [Agonists]
A number of peptide and orally-active nonpeptide
agonists and antagonists have been investigated. Recent
research to find coupling-selective ligands and to design
bivalent ligands found that potent selective agonists are
[Phe2
, Orn8
]VP and F-180 (V1a agonists), d[Cha4
]AVP
(V1b agonist), and dDAVP and dVDAVP (V2a agonists).
The selectivities vary between human and rat receptors. | [Antagonists]
Potent selective antagonists are d(CH2)5[Tyr(Me)2
]
AVP and SR49059 (V1a antagonists); SSR149415 (V1b
antagonist); and OPC31260, OPC41061, and SR121463
(V2 antagonists). | [Receptors]
Three functional receptors have been identified (V1aR,
V1bR, and V2aR). In humans, the length (aa residues),
chromosomal location, and number of exons are as follows: V1aR, 418, 12q14-15, two exons; V1bR, 424, 1q32,
two exons; and V2aR, 371, Xq28, three exons. AVP binds
to three receptors with high affinity (Kd<2 nM). GPCRs
including VP and OT receptors may also function as
homo/hetero-dimers/oligomers. | [Biological functions]
The most prominent function of AVP is the antidiuretic action through V2aR/aquaporin-2 (AQP2) in the inner
medullary collecting duct of the kidney. AVP administration also induces a large increase in blood pressure. This
effect is caused by vascular contraction, basoreflex, renin
production, and aldosterone and glucocorticoid releases
from the adrenal gland, all of which are mediated via
V1aR. In addition, adrenocorticotropic hormone
(ACTH) and catecholamine release via V1bR and body
fluid retention via V2aR contribute to blood pressure regulation. In the pituitary, AVP synergistically controls the
secretion of ACTH with corticotropin-releasing hormone
(CRH). AVP also regulates metabolic function via V1aR
and V1bR. In the central nervous system,
AVP is involved in social behaviors, learning, memory,
aggression, anxiety, depression, water and food intake,
circadian rhythm, and thermoregulation. | [Regulation of synthesis and release]
The synthesis and release of AVP are stimulated by the
elevation of plasma osmolality and decreases in blood volume and blood pressure. Magnocellular AVP neurons
receive signals of various neurotransmitters and neuromodulators from the circumventricular organs and medulla, including GABA, noradrenaline, dopamine, glutamic acid, atrial natriuretic peptide, and angiotensin II. In
the parvocellular PVN, adrenalectomyincreases AVP synthesis while elevation is restored by dexamethasone
administration. Direct regulation by androgen and estrogen is shown in the BNST and amygdala. Various peptide
hormones (such as galanin, enkephalin, neuropeptide Y,
vasoactive intestinal peptide, dynorphin) are colocalized
in AVP neurons, and are involved in the regulation of
AVP and OT release. | [Clinical implications]
Approximately 90% of CNDI patients are males with
mutations in the V2aR gene on the X-chromosome.
Besides CNDI, gain-of-function mutations in V2aR cause
nephrogenic syndrome of inappropriate antidiuresis
(NSIAD), characterized by excessive sodium excretion,
low plasma sodium concentration, and low plasma
osmolality. The syndrome of inappropriate antidiuretic
hormone secretion (SIADH) is typically associated with
measurably elevated AVP levels and consequent V2aR
hyperactivity.Due to the equimolar secretion with
VP, plasma copeptin levels can be a prognostic marker
in acute diseases, and a promising marker in the diagnosis of VP-related disorders. |
| Hazard Information | Back Directory | [Description]
Vasopressin, also called antidiuretic hormone (A D H) or arginine vasopressin
(AVP), is another hormone key to water homeostasis and blood pressure
regulation. A rginine vasopressin is produced in the neurones of the
hypothalamus and stored in vesicles within the posterior pituitary.
Vasopressin is released in response to increased blood osmolality detected
by hypothalamic osmoreceptors; systemic hypotension or hypovolaemia
detected by cardiopulmonary baroreceptors of the great veins and atria; or
angiotensin I I acting on the hypothalamus. | [Uses]
Hormone (antidiuretic). | [Brand name]
Pitressin
(Parke-Davis). | [Biological Functions]
Human vasopressin, or Arg-vasopressin, is chemically very similar to oxytocin and therefore
sometimes is referred to as [Phe3, Arg8]oxytocin. The physiological role of
vasopressin is the regulation of water reabsorption in the renal tubules (an antidiuretic action,
thus often referred to as the antidiuretic hormone). In high doses, vasopressin promotes the
contraction of arterioles and capillaries, resulting in an increase in blood pressure, thus the
name vasopressin. An inadequate output of pituitary antidiuretic hormone can cause diabetes
insipidus, which is characterized by the chronic excretion of large amounts of pale urine and
results in dehydration and extreme thirst. | [Clinical Use]
The nonapeptide vasopressin is well known for its role on fluid metabolism, but it also is a
key regulator of the HPA axis. Stress stimulates the release of vasopressin in the pituitary gland, where it
strongly potentiates the effects of CRF on adrenocorticotropic hormone release. These findings suggest that HPA axis dysregulation in depression might be associated with
the development of centrally acting vasopressin receptor antagonists for the treatment of depression.
|
|
|






