| Identification | Back Directory | [Name]
Polyacrylamide | [CAS]
9003-05-8 | [Synonyms]
OF
PHⅢ
PAM
j100
p250
PAAm
TAF1
BA2R
CCG1
CCGS
TAF2A
NSCL2
PHIII
paa-1
k-pam
ap273
et597
pamid
dow164
paa70l
pam-50
N-TAF1
PAD235
PAM/PHⅢ
dowj100
cytame5
SPAR-50
reten420
dowet597
flygtolgb
DYT3/TAF1
PNIPAM-NH2
PNIPAM-NHS
aminogenpa
polystolon
polystoron
sumireza17
sumireza27
sursolanp5
polyhall27
polyhall402
AQUACIDE IV
versicolw11
sanpolya520
porisutoron
cyanamerp35
gelamide250
PNIPAM-COOH
himolocok507
himolocss200
cyanamerp250
praestol2800
pam[polymer]
solvitose433
stokopold2624
magnaflocr292
j100[polymer]
p250[polymer]
PolyacryaMide
GenElute?-LPA
PoIyacrylamide
POLYACRYLAMIDE
diaclearma3000h
GENELUTE(TM)-LPA
ACRYLAMIDE RESIN
paa1[homopolymer]
k4[acrylicpolymer]
ACRYLAMIDE POLYMER
acrylamide,polymers
Poly(2-Propenamide)
americancyanamidkpam
POLYACRYLAMIDE RESIN
LINEAR POLYACRYLAMIDE
americancyanamidp-250
acrylamidehomopolymer
Acrylamide urea TBE
KAT4, GST tagged human
Polyacrylamide colloid
Polyacrylamide AK-618-0
Polyacrylamide solution
Acrylamide gel solution
Polyacrylamide Mn 40,000
Polyacrylamide,hydrolyzed
2-Propenamide homopolymer
Acrylamide resin (low M.Wt.
Polyacrylamide absorbent Gel
Acrylamide resin (high M.Wt.)
Polyacrylamide aqueous solution
diacetone acrylamide 235 solution
Polyacrylamide dry powder,anionic
Poly(acrylamide) average Mn 150,000
POLYACRYLAMIDE NONIONIC WATER-SOLUBL
ACRYLAMIDE GEL SOLUTION FOR MOLECULAR
Polyacrylamide—PAM(Anionic and Cationic)
Acrylamide Polymer
Acrylamide Polymer
PHIII
ACRYLAMIDE RESIN (LOW M.WT., LOW CARBOXYL CONTENT)
Poly(N-isopropylacrylamide), maleimide terminated
Polyacrylamide, nicht ionisch mit einem Restmonomergehalt <0,1 %
POLYACRYLAMIDE, AVERAGE MW CA. 1,500, 50 WT. % SOLUTION IN WATER
Poly(acrylamide), granular, non-ionic, approx. MW. 5 to 6.000.000
Poly(N-isopropylacrylamide), maleimide terminated average Mn 2,000
Poly(N-isopropylacrylamide), maleimide terminated average Mn 5,500
Poly(acrylamide), granular, non-ionic, average M.W. 5 to 6.000.000
POLYACRYLAMIDE, AVERAGE MW CA. 10,000, 5 0 WT. % SOLUTION IN WATER
Poly(acrylaMide), granular, non-ionic, approx. M.W. 5 to 6.000.000 5GR
Poly(N-isopropylacrylamide), carboxylic acid terminated average Mn 5,000
Poly(N-isopropylacrylamide), carboxylic acid terminated average Mn 7,000
Poly(N-isopropylacrylamide-co-methacrylic acid-co-octadecyl acrylate)
AcrylaMide PolyMer (Mw.=400,000-800,000) (containing sMall aMounts of forMalin as fungicide) (10% in Water)
Acrylamide Polymer (Mw.=600,000-1,000,000) (containing small amounts of formalin as fungicide) (10% in Water) | [EINECS(EC#)]
231-545-4 | [Molecular Formula]
C3H5NO | [MDL Number]
MFCD00084392 | [MOL File]
9003-05-8.mol | [Molecular Weight]
71.0779 |
| Chemical Properties | Back Directory | [Appearance]
white to faintly yellow granules | [Melting point ]
>300 °C | [density ]
1.189 g/mL at 25 °C
| [Tg]
165°C | [refractive index ]
n20/D 1.452
| [Fp ]
>230 °F
| [storage temp. ]
Refrigerator | [solubility ]
Water | [form ]
Granules | [color ]
White to faintly yellow | [Odor]
odorless | [Stability:]
Stable. Incompatible with strong oxidizing agents, aluminium, copper, iron, iron salts | [Water Solubility ]
SOLUBLE | [Uses]
polyacrylamide is a binder, film former, and fixative with greater use in hair and nail than in skin care preparations. It is used in some hand and body lotions and cleansing creams. | [EPA Substance Registry System]
2-Propenamide, homopolymer(9003-05-8) |
| Hazard Information | Back Directory | [Definition]
ChEBI: A macromolecule composed of repeating 1-carbamoylethylene units. | [Preparation]
Polyacrylamide resembles poly(acrylic acid) and poly(methacrylic acid)
in being water-soluble and, as with those polymers, it is mainly
this property which results in some limited commercial utilization.
Polyacrylamide is prepared by free radical polymerization, using techniques essentially similar to those described for poly(acrylic acid) and poly(methacrylic acid):
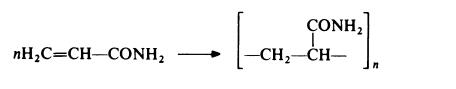
It may be noted that whereas this reaction gives a vinyl polymer, a different
type of polymer is obtained when polymerization is initiated by a strong base.
In this case, a polyamide (nylon 3) is formed. Active initiators for this type of
polymerization include alkoxides (RO-) and reaction probably takes place
according to the following scheme, which involves the rearrangement of a
carbanion to a more stable amide anion:
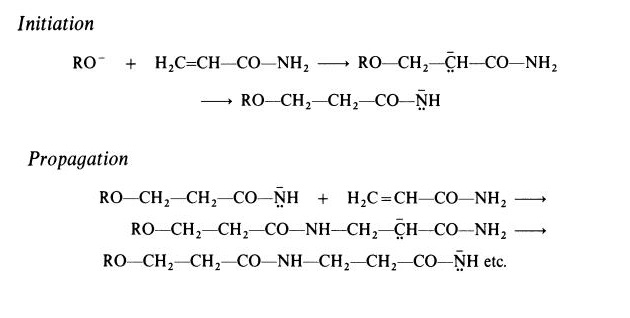
Polyacrylamide is a hard, brittle material. It is readily soluble in cold water
but solubility in organic compounds is generally very limited. The polymer
undergoes reactions characteristic of the amide group; for example, alkaline
hydrolysis introduces carboxylic groups and reaction with formaldehyde
gives methylol groups.
Polyacrylamide has found use as a ftocculant in the processing of minerals
and in water treatment. Copolymers of acrylamide and acrylic acid are used
to increase the dry strength of paper. | [General Description]
Intelligent Swelling/Collapsing copolymer that can be used as a temperature- and pH-sensitive material. | [Biochem/physiol Actions]
Polyacrylamide is a water-soluble polymer made up of acrylamide subunits. It increases the viscosity of water and facilitates the flocculation of particles present in water. | [Solubility in organics]
Ethylene glycol, glycerol, lactic acid, water | [Properties and Applications]
|
INDEX
|
Results
|
|
Appearance
|
White powder / Granular
|
|
Molecular Weight
|
5000000-30000000
|
|
Degree of Hydrolysis
|
6-45%
|
|
Solid Content
|
89% min
|
|
Dissolving Time
|
60 minutes max
|
|
Effective pH Range
|
5~14
|
|
Residual Monomer
|
0.1% max
|
|
| Questions And Answer | Back Directory | [High polymer]
Polyacrylamide, also briefly referred as PAM, is commonly a polymer with acrylamide monomers bonded connected by end to end configuration; it is a hard glassy solid at room temperature. Because of the difference in production methods, the products can be white powder, translucent beads and flaky like. Its density is 1.302 g/cm3 (23 °C) with glass transition temperature being 153 °C and softening temperature being 210 °C. It has good thermal stability and is soluble in water; its aqueous solution is clear and transparent with its viscosity increasing with increased molecular weight of the polymer, and also having a logarithmic relationship with the change in concentration of the polymer. Except for a few solvent such as acetic acid, acrylic acid, ethylene glycol, glycerol and formamide, it is generally insoluble in organic solvents.
It is formed by the polymerization of free acrylamide monomer radical. It can be produced by several methods such as solution polymerization, inverse emulsion polymerization, suspension polymerization and solid state polymerization. Demanded product should have controllable molecular weight, good water solubility and with little residual monomers.
PAM is one of the most widely used water-soluble polymer species with a large number of pendant amide groups presenting on its molecular backbone. Amide group has a high chemical activity which can forms a series of derivatives with many kinds of compounds. Polyacrylamide has effects of flocculation, thickening, drag reduction, adhesive, colloidal stabilizing, filming and preventing scale. It is widely used in papermaking, mining, coal washing, metallurgy, oil exploitation and other industrial sectors and is also a important chemical for water treatment.
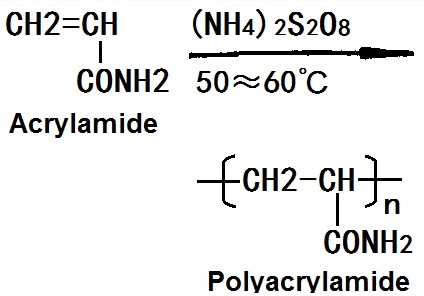
Figure 1 The synthetic route of polyacrylamide.
| [Chemical Properties]
Polyacrylamide is relatively stable to heat with its solid only being softened at 220~230 °C and its solution subjecting to significant degradation only at above 110 °C. Polyacrylamide is insoluble in benzene, toluene, xylene, gasoline, kerosene, diesel fuel, but soluble in water. Polyacrylamide can react with alkaline with partial hydrolysis of polyacrylamide. It will have imidization reaction in strongly acidic (pH≤2.5) which will reduce its solubility in water. It can be cross-linked by the poly-nuclear olation complex ion formed between aldehyde (such as formaldehyde) and high metal (such as aluminum, chromium, zirconium, etc.) and is easy to be degraded by the action of the mechanical and (or) oxygen. In oil exploitation, it is mainly used as oil displacement agent, water blocking agent, profile control agent, thickener, drag-reducing agent, water treatment agent.
| [Physical properties]
Solubility in water: upon rapid mechanical stirring, polyacrylamide is easily soluble in cold water form a transparent adhesive solution. Increasing the temperature does not affect its solubility and only affects its dissolution when the concentration is increased to a high viscosity.
Solubility in Other Solvents: polyacrylamide has a over 1% solubility in solvents such as glycerol, ethylene glycol, formaldehyde, acetic acid and lactic acid (these materials may be used as the plasticizer for laminating polyacrylamide). However, it can only be swelled without being dissolved in solvents such as propionic acid, propylene glycol; it is also not soluble in solvent such as acetone and hexane.
Stability: polyacrylamide has a moderate hygroscopic property, if not exposed to position of high temperatures, the powdered polyacrylamide can subject to long-term storage. For liquid polyacrylamide, when its concentration is greater than 17%, it can be stored for more than one year with no significant change in the solution viscosity. In the pH range of 3 to 9, it can maintain a good degree of stability; at high pH, ??the viscosity will be increased gradually.
Miscibility: in generally used concentration, polyacrylamide has miscibility with most water-soluble natural or synthetic resins, latex systems, and most of the salts. Polyacrylamide can also quickly miscible with non-ionic, cationic and anionic surfactants, though with certain surfactants affecting the viscosity.
Viscosity: The viscosity of polyacrylamide solution has a linear correlation with its molecular weight; in addition, the higher the temperature, the lower the viscosity.
Intrinsic viscosity: the increase of the molecular weight of polyacrylamide will cause increased intrinsic viscosity.
Ion property: the carboxyl group in long-chain yields anionic polyacrylamide; the amino group yields cationic version. Because of the existence of amino group or carboxyl group in the long-chain of polyacrylamide, it is easy for flocculation when encountering aluminum ions.
Retention property: The retention trend of polyacrylamide is similar with that of rosin soap with the former one having a high retention rate.
Toxicity: Polyacrylamide itself is non-toxic, but if it contains polymerized monomers (a double bond), it would be toxic to humans. For this reason, upon the completion of its preparation, a certain amount of sodium bicarbonate should be added to remove residual monomers.
The above information is edited by the chemicalbook of Dai Xiongfeng.
| [Anionic and cationic polyacrylamide]
Polyacrylamide is non-toxic and with a high molecular weight and is highly water soluble, and can introduce a variety of ionic groups for adjusting the molecular weight to obtain specific performance; it has good adhesion to many solid surface and dissolved substances, and can adhere or bridge the suspended particles dispersed in the solution for flocculation of them which is easy for filtration and separation.
Anionic polyacrylamide can be used as a cytoplasm additive in the paper industry with better retention and drainage effect. It has a particularly dispersing effect for long-fiber pulp when its molecular weight is greater than 3.5 million. In addition, it can also be used as a water treatment agent. In petroleum industry, it can be used as oilfield mud additives, thickeners, and settling agents. In coal industry, it is used as coal-washing additive.
Anionic polyacrylamide has generally two ways of preparation, one is copolymerization, which was prepared by the copolymerization of acrylamide and acrylic acid or sodium acrylate aqueous solution; the other is the chemical conversion method, that is, from partial alkaline hydrolysis of polypropylene amide, or prepared by alkaline hydrolysis of poly-acrylonitrile. Here is copolymerization method whose procedure is simple and easy to control. The specific method is by mixing the 20% acrylamide and sodium acrylate aqueous solution in certain ratio. 200 parts of this mixed monomer were added about 1 part of 1% EDTA solution, and then add it into 460 parts of deionized water; then add 2-3 parts of both 5% ammonium persulfate and sodium hydrogen sulfite solution under continuous flow of nitrogen, stir for 3-4 hours at 40~50 °C.
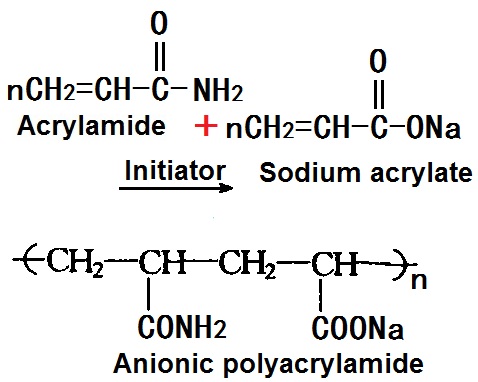
Figure 2 The synthetic route of Anionic polyacrylamide.
Cationic polyacrylamide is cationic, and thus having strong flocculation and absorption ability on the anionic material such as cellulose. As retention aids in the paper industry, it can increase filler and fines retention; as a filter aid, it has a strong flocculation effects on slurry and can accelerate the filtration accelerated of the wet in the wire section layer of paper machine; as a neutral sizing precipitating agent, it can partly substitute alum, and making rosin gum be precipitated and adhered between the fibers at higher pH. Ti can also accelerate the settlement of fibers in the white water and the flocculation of the suspended solids in pulp waste water, and thus can be used for waste water processing.
Preparation: the polymerization of acrylamide aqueous solution is a common method for preparing polyacrylamide. According to the trigger mode, there are different methods such as thermal initiator and oxidation-reduction induction. Polymer prepared by persulfate thermal initiating has relative small molecular weight at about 20 to about 1,000,000. On the other hand, polymer produced with oxidation-reduction method usually have relative high molecular weight polymer, up to about 300 to 400 million. For the application of retention and drainage aid in paper industry, it is better to apply polyacrylamide with higher molecular weight. The following is oxidation-reduction triggering polymerization. The resulting polymer, under alkaline conditions, is subject to Hofmann degradation reaction of amide in the sodium hypochlorite solution to obtain the acrylamide-amino-ethylene copolymer containing about 1% of free amino group.
Finally, neutralize with hydrochloric acid and further adjust to pH 5.5 to 6. The resulting product has a cationic property, and is a kind of cationic polyacrylamide with good application performance and low cost.
| [Polyacrylamide gel electrophoresis]
Polyacrylamide gel electrophoresis is an important means of DNA, RNA and protein analysis and separation. Ions and charged molecules mobilize in electric field with the mobility rate being related to their molecular size and shape, the strength of the molecular charge, the current strength and the resistance of the media to the current and therefore forming separate bands.
1. DNA polyacrylamide gel electrophoresis: PAGE can separated the substances according to their differences on charge, molecular size and the shape, thus having a molecular sieve effect as well as an electrostatic effect; it has a higher resolution than agarose gel electrophoresis and is suitable for the isolation of DNA oligonucleotides and its sequence analysis. Compared with agarose gel electrophoresis, it has the following advantages:
(1)A stronger distinguishing ability, though the maximum fragment is 500 times as long as the smallest fragment length, they can still be well separated;
(2) Capable of loading a higher amount of DNA than agarose gel;
(3)The purity recovered from PAGE recovered is high which is suitable for demanding experiments.
2. RNA polyacrylamide gel electrophoresis (gel electrophoresis with vertical plate): it can isolate and analyze several different RNA samples or large doses of RNA samples simultaneously.
3. Protein polyacrylamide gel electrophoresis:
(1)The basic principle of SDS denaturing in-continuous polyacrylamide gel electrophoresis is based on differences in the molecular weight of the protein, SDS is an anionic surfactant, capable of binding with the hydrophobic portion of the protein thus making the protein bring a large number of anions SDS. Protein molecules thus bring a lot of negative charge which is far beyond its original charge. Thus the difference between the charges on different proteins has no significant effect. SDS can also make protein structure become loose with converged shape; the mobility rate of SDS-protein complex is only related to molecular weight. PAGE not only has molecular sieve effect but also has concentrated effect. Owing to the effect of the discontinuous pH gradient, the sample is compressed into a narrow band, thereby improving the separation efficiency.
(2)Discontinuous non-denaturing polyacrylamide gel electrophoresis: the operation process such as materials, glue and electrophoresis and exactly the same as SDS-PAGE. The only difference is that all reagents are free of SDS.
(3)Continuous non-denaturing polyacrylamide gel electrophoresis: electrophoresis system is to use a continuous pH value, and one layer of gel, and one kind of buffer system. The operation is the same as SDS-PAGE.
| [The maximum amount for food additives as maximal allowable residue]

| [Uses]
1. It is used as a flocculant in water treatment industry. Also used in petroleum geology drilling configuration for removing non-dispersing low solid phase mud.
2. It can be used as setting agent in sugar industry settling agent (sugar co-agent); film formers.
3. It can be used as a soil conditioner, flocculants, and can be used in textile and paper sizing reinforcement.
4. It can be used at coal field, oil field and flocculant agents.
5. It can be used as efficient flocculants for neutral and alkaline medium, and can be used as drilling mud additives.
6. It can also be used as oilfield mud additives, sewage treatment agent, and for textile sizing, paper reinforcement.
7. PAM is an important water-soluble polymer, and also has various values effects such as flocculation, thickening, cleavage resistant, reducing resistance, and dispersing properties. These properties are biased according to the difference of the derivative ions. Therefore, it has wide application in various fields such as oil exploration, mineral processing, coal washing, metallurgy, chemicals, paper, textile, sugar, medicine, environmental protection, building materials, and agricultural production.
8. It can be used as the flocculant for water-based drilling fluid which can improve the rheological properties of the drilling fluid, reducing friction.
9. It is widely used in petrochemical, metallurgy, coal, mineral processing and textile and other industrial sectors, and is also used as precipitation flocculant, oil field water thickeners, drilling mud treatment agent, textile pulp, paper reinforcing agent, fiber modifier, soil conditioners soil stabilizing agent, fiber paste, resin finishing agents, synthetic resin coatings, adhesives, and dispersing agents.
| [Production methods]
1. Acrylonitrile is hydrated to obtain acrylamide with copper as the catalyst, and further polymerized into polyacrylamide in the action of K2S2O8. Copper-aluminum alloy is converted into catalyst by alkali washing and pour into the hydration reactor. The raw material of acrylonitrile is pumped to storage tanks and then into the measuring tank, pour the water subjecting to post-ion exchange process into the measuring tank and then pump raw materials through the pre-heater continuously into the hydration reactor in proportion; control at 85-125 °C for hydration reaction to obtain aqueous solution of acrylamide with the remaining acrylonitrile recovered through flash column and condenser and further flowed back into the water metering tank for recycling usage and the acrylamide solution flowing from flash tank into the tank; Pump it into high slot to the resin exchange column to become 7-8% monomer after entering into tank, send it to the polymerization reactor to produce gel-like polyacrylamide gel package which is the final product.
2. Colloidal polyacrylamide: add 1 200 kg of deionized water into the hydrolysis reactor, add under stirring of acrylonitrile, 0.3 kg of aluminum hydroxide, cupric hydroxide for complex catalysis, and have hydrolysis reaction at 85~125 °C. After completion of the reaction, distill off the unreacted monomer acrylonitrile. Prepare a 7% to 8% acryloyl aqueous solution, add polymerization vessel and have polymerization reaction upon the triggering of ammonium persulfate.
High molecular weight-polyacrylamide; hydrolyze acrylonitrile at 110~140 °C, 0.3 MPa into acrylamide. Add PAGE into the polymerization vessel containing deionized water, and have reaction for 8 to 24 h in the triggering of 50 mg/kg of ammonium persulfate. Then, it is hydrolyzed into the final product under alkaline conditions and at 70~80 °C.
3. Acrylonitrile is first catalyzed into acrylamide, and then further polymerized into polyacrylamide in the presence of K2S2O8.
4. Add measured acrylonitrile into the reaction vessel; further add a catalytic amount of copper-based catalyst. Stir and warm up to 85~120 °C. The reaction pressure was controlled at 0.29~0.39 MPa. In continuous operation, the feed content was controlled at 6.5% with empty velocity of about 5h-1. The obtained acrylamide was then transferred polymerization vessel; add a certain amount of deionized water. Have the polymerization reaction in the triggering of potassium persulfate; add an appropriate amount of sodium bisulfite at 10 mins after the start of the reaction. Gradually heat to 64 °C, cool the reaction mixture, and have reaction at about 55 °C for 6h. Remove the unreacted monomer at vacuum (80 °C) under reduced pressure to obtain the finished product.
|
|
|