| Identification | Back Directory | [Name]
Vonoprazan FuMarate | [CAS]
881681-01-2 | [Synonyms]
Vonoprazan FuMarate###NA
1-(5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)-N-methylmethanamine fumarate
5-(2-Fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-1H-pyrrole-3-methanamine (2E)-2-butenedioate | [EINECS(EC#)]
1592732-453-0 | [Molecular Formula]
C21H20FN3O6S | [MOL File]
881681-01-2.mol | [Molecular Weight]
461.463 |
| Chemical Properties | Back Directory | [storage temp. ]
Store at -20°C | [solubility ]
DMSO: 50 mg/mL (108.35 mM) | [form ]
Solid | [color ]
White to Off-White | [InChIKey]
ROGSHYHKHPCCJW-WLHGVMLRSA-N | [SMILES]
C(/C(=O)O)=C\C(=O)O.S(C1=CN=CC=C1)(N1C=C(CNC)C=C1C1C=CC=CC=1F)(=O)=O |
| Hazard Information | Back Directory | [Chemical Properties]
Vonoprazan fumarate is white to nearly white crystals or crystalline powder which melts at 194.8°C . It can be dissolved in dimethyl sulfoxide, but has limited solubility
in N,N-dimethylacetamide, N,N-dimethylformamide, methanol, and water. It
is only slightly soluble in ethanol (99.5%), and has very low
solubility in 2-propanol, acetone, 1-octanol, and acetonitrile. | [Uses]
Vonoprazan Fumarate (CAS# 881681-01-2) is a useful research chemical and a ATPase potassium-competitive acid blocker. | [benefits]
Compared with traditional irreversible proton pump inhibitors (such as omeprazole, esomeprazole, etc.), Vonoprazan fumarate advantages:
① The onset of action is good, and the maximum acid-suppressing effect will be achieved on the first day of administration;
② Oral administration, not affected by gastric acid damage;
③It can improve the phenomenon of acid breakthrough at night. | [General Description]
Vonoprazan fumarate (TAK-438) is a new oral anti-gastric acid drug jointly launched by Takeda Pharmaceutical and Otsuka Pharmaceutical. It can be used for the treatment of erosive esophagitis, gastric ulcer and duodenal ulcer. | [Mechanism of action]
Vonoprazan fumarate is a potassium ion (K+) competitive acid blocker (P-CAB), which is a reversible proton pump inhibitor. It can inhibit the combination of K+ and H+-K+-ATPase (proton pump), so that secretion of gastric acid is terminated. Vonoprazan fumarate has a strong and lasting inhibitory effect on gastric acid secretion. | [Synthesis]
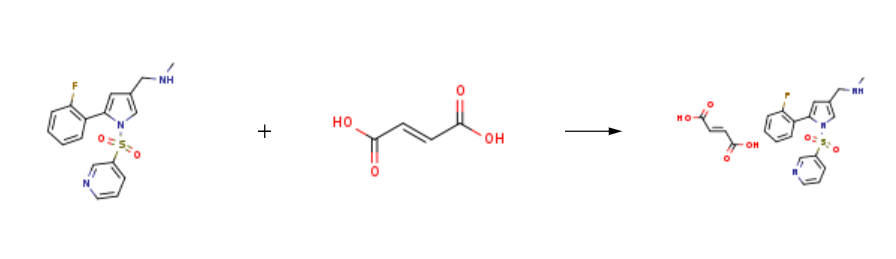
Vonoprazan FuMarate is prepared by the reaction of vonoprazan and (2E)-but-2-enedioic acid. The steps are as follows:
Step 1: (2E)-but-2-enedioic acid With sodium hydroxide In water at 50 - 60℃.
Step 2: vonoprazan In water; toluene at 25 - 35℃; Reagent/catalyst.
Add 730g of water and 73g of fumaric acid to a 2L reaction flask at 5060, stir and disperse, control the temperature between 5060, add 25g of hydrogen hydroxide dropwise to the fumaric acid suspension The solution of sodium and 300 g of water was gradually dissolved in the system during the dropwise addition, and the aqueous solution of compound was obtained and kept for use.Between 25 and 35°C, take half of the toluene solution of compound obtained in the previous step and put it into a 5L reaction flask, and add the aqueous solution of the above compound dropwise to it. During the dropwise addition, the system has a white solid precipitation. After the dropwise addition was completed, keep stirring for 2-4 hours, filter, and dry the filter cake at 50°C to obtain 131.32 g of white solid, the HPLC purity was 99.96%, and the yield was 94%.
 |
|
|