| Identification | Back Directory | [Name]
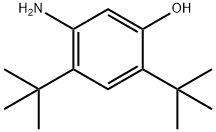
5-AMino-2,4-di-tert-butylphenol | [CAS]
873055-58-4 | [Synonyms]
VX-770 intermediate
4,6-dichloropyridazin-4-ol
5-AMino-2,4-di-tert-butylphenol
5-Amino-2,4-ditertiarybutyphenol
5-Amino-2,4-bis(2-methyl-2-propanyl)phenol
Phenol, 5-amino-2,4-bis(1,1-dimethylethyl)-
2,4-Di-tert-butyl-5-aMinophenyl Methyl carbonate
5-amino-2,4-di-tert-butylphenol hydrochloride (base) | [Molecular Formula]
C14H23NO | [MDL Number]
MFCD11052607 | [MOL File]
873055-58-4.mol | [Molecular Weight]
221.34 |
| Chemical Properties | Back Directory | [Boiling point ]
319.7±30.0 °C(Predicted) | [density ]
0.990±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,2-8°C | [pka]
11.63±0.23(Predicted) | [InChI]
InChI=1S/C14H23NO/c1-13(2,3)9-7-10(14(4,5)6)12(16)8-11(9)15/h7-8,16H,15H2,1-6H3 | [InChIKey]
BKSDHPHOWDUJNB-UHFFFAOYSA-N | [SMILES]
C1(O)=CC(N)=C(C(C)(C)C)C=C1C(C)(C)C |
| Hazard Information | Back Directory | [Uses]
5-Amino-2,4-di-tert-butylphenol is an intermediate used in the preparation of ivacaftor via condensation of oxo dihydroquinoline carboxylic acid with amino ditert butylphenol. | [Synthesis]
To a refluxing solution of 2,4-di-tert-butyl-5-nitro-phenol (1.86 g, 7.40 mmol) and ammonium formate (1.86 g) in ethanol (75 mL) was added Pd-5% wt on activated carbon (900 mg). The reaction mixture was stirred at reflux for 2 h, cooled to room temperature, and filtered through Celite. The Celite was washed with methanol, and the combined filtrates were concentrated to yield 5-amino-2,4-di-tert-butyl-phenol as a grey solid (1.66 g, quant.).
|
|
|