| Identification | Back Directory | [Name]
Bis[n-(p-toluenesulfonyl)]sulfodiimide | [CAS]
851-06-9 | [Synonyms]
bis[n-(p-toluenesulfonyl)]sulfodiimide
Benzenesulfonamide, N,N'-λ4-sulfanetetraylbis[4-methyl- | [Molecular Formula]
C14H14N2O4S3 | [MOL File]
851-06-9.mol | [Molecular Weight]
370.46 |
| Chemical Properties | Back Directory | [Melting point ]
119-120 °C | [Boiling point ]
523.3±53.0 °C(Predicted) | [density ]
1.40±0.1 g/cm3(Predicted) | [solubility ]
sol benzene, toluene, chloroform, and methylene chloride | [color ]
bright yellow solid |
| Hazard Information | Back Directory | [Uses]
Bis[n-(p-toluenesulfonyl)]sulfodiimide can act as an enophile in ene reactions and as a dienophile in Diels-Alder reactions; participates in [2 + 2] and [3 + 2] cycloaddition reactions; cycloadds to ynamines. | [Synthesis]
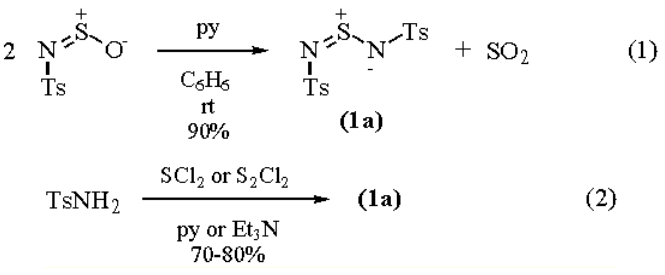
A convenient in situ method for the synthesis of bis[N-(p-toluenesulfonyl)]sulfodiimide is by treating N-Sulfinyl-p-toluenesulfonamide with dry Pyridine in
benzene (eq 1). An alternative procedure is the reaction of SCl2 or S2Cl2 with p-toluenesulfonamide in the
presence of a base such as pyridine or triethylamine (eq 2).
 | [storage]
Extremely sensitive to moisture and should be stored in a desiccator and handled only in a dry box or glove bag. Typically the title compound is generated and used in situ. |
|
|