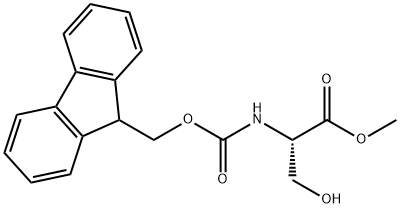
| Identification | Back Directory | [Name]
FMOC-SER-OME | [CAS]
82911-78-2 | [Synonyms]
FMOC-SER-OME
Fmoc-L-Ser-OMe
FMOC-SERINE-OME
FMOC-L-SERINE METHYL ESTER
Fmoc-L-β-hydroxyalanine methyl ester
Fmoc-L-serine methyl ester≥ 99.5% (HPLC)
(9H-Fluoren-9-yl)MethOxy]Carbonyl Ser-OMe
(S)-Fmoc-2-Amino-3-hydroxypropionic acid methyl ester
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-L-SERINE METHYL ESTER
(S)-Methyl 2-((((9H-fluoren-9-yl)Methoxy)carbonyl)aMino)-3-hydroxypropanoate | [Molecular Formula]
C19H19NO5 | [MDL Number]
MFCD00672334 | [MOL File]
82911-78-2.mol | [Molecular Weight]
341.36 |
| Chemical Properties | Back Directory | [Boiling point ]
579.4±45.0 °C(Predicted) | [density ]
1.285±0.06 g/cm3(Predicted) | [storage temp. ]
-15°C | [form ]
Solid | [pka]
10.07±0.46(Predicted) | [color ]
White to off-white |
| Hazard Information | Back Directory | [Chemical Properties]
White to off-white powder | [Uses]
Fmoc-Ser-OMe (Fmoc-L-Ser-OMe) is a hydroxylated L-amino acid protected with a 9-fluorenylmethyloxycarbonyl (Fmoc) group. Fmoc-Ser-OMe involves in chlorophyll–amino acid conjugates synthesis, and acts as a chromo/fluorophores modified protein and emits visible to near-infrared lights efficiently. Fmoc-Ser-OMe glycosylates and produces small mucin-related Olinked glycopeptides, as an alcohol acceptor[1][2]. | [References]
[1] Tamiaki H, et al. Synthesis of chlorophyll-amino acid conjugates as models for modification of proteins with chromo/fluorophores. Bioorg Med Chem. 2014 Feb 15;22(4):1421-8. DOI:10.1016/j.bmc.2013.12.059
[2] K?rkk?inen TS, et al. Iodine-mediated glycosylation en route to mucin-related glyco-aminoacids and glycopeptides. Carbohydr Res. 2008 Jul 21;343(10-11):1830-4. DOI:10.1016/j.carres.2008.03.034 |
|
|