| Identification | Back Directory | [Name]
ISOXABEN | [CAS]
82558-50-7 | [Synonyms]
el107
NA 8318
Isoaxben
ixoxaben
FLEXIDOR
ISOXABEN
benzamizole
ISOXABEN STANDARD
ISOXABEN PESTANAL
ISOXABEN PESTANAL, 250 MG
Isoxaben @100 μg/mL in MeOH
ISOXABEN ANALYTICAL STANDARD
Isoxaben@1000 μg/mL in Methanol
Isoxaben Solution in Methanol, 100μg/mL
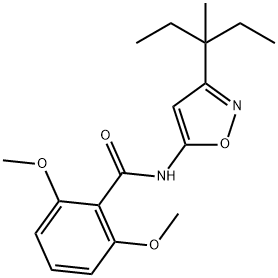
N-(3-(1-ethyl-1-methylpropyl)-5-isoxazoyl)-2,6-dimethoxybenzamide
n-(3-(1-ethyl-1-methylpropyl)-5-isoxazolyl)-2,6-dimethoxybenzamide
2,6-dimethoxy-n-(3-(1-ethyl-1-methylpropyl)-5-isoxazolyl)-benzamid
2,6-dimethoxy-n-(3-(1-ethyl-1-methylpropyl)-5-isoxazolyl)benzamide
N-[3-(1-Ethyl-1-methylpropyl)isoxazol-5-yl]-2,6-dimethoxybenzamide
Benzamide, N-3-(1-ethyl-1-methylpropyl)-5-isoxazolyl-2,6-dimethoxy-
N-(3-(1-ethyl-1-methylpropyl)-1,2-oxazol-5-yl)-2,6-dimethoxybenzamide | [EINECS(EC#)]
407-190-8 | [Molecular Formula]
C18H24N2O4 | [MDL Number]
MFCD00078687 | [MOL File]
82558-50-7.mol | [Molecular Weight]
332.39 |
| Chemical Properties | Back Directory | [Melting point ]
175-179 °C | [Boiling point ]
469.52°C (rough estimate) | [density ]
1.2149 (rough estimate) | [refractive index ]
1.5700 (estimate) | [storage temp. ]
0-6°C | [form ]
neat | [pka]
11.44±0.70(Predicted) | [EPA Substance Registry System]
Isoxaben (82558-50-7) |
| Hazard Information | Back Directory | [Uses]
Isoxaben is a herbicide residue in tea and other plants. A cellulose biosynthesis inhibitor in plants. | [Uses]
Isoxaben is used primarily for preemergence control of
annual broadleaf weeds. Isoxaben is usually applied to
soil either with light incorporation or before application
of water (at least 0.5 in) within 3 weeks. As is the case
with DCB, it is most effective on weed seedlings before
emergence. | [Definition]
ChEBI: A benzamide obtained by formal condensation of the carboxy group of 2,6-dimethoxybenzoic acid and the amino group of 3-(3-methylpentan-3-yl)-1,2-oxazol-5-amine. | [Pharmacology]
Although isoxaben is readily absorbed through the
root system, foliar absorption and translocation is poor.
Reduced root absorption may be partly responsible for
the tolerance of some dicot species to isoxaben, although
differences in the site of action appears to be the major
contributing factor in tolerance (15).Up to 50% of absorbed
isoxaben is metabolized within 6 days following root application
(16). Differences in metabolism cannot explain
the selectivity of isoxaben between tolerant and susceptible
species (17,18). Metabolism of isoxaben involves
hydroxylation of the propyl side group and glucosylation.
2,6-dimethoxybenzamide is found as a minor metabolite
(19). Isoxaben prevents the germination and growth of
seedlings before emergence, by inhibiting cell division.
The primary mode of action of isoxaben is inhibition of
cellulose biosynthesis, although the exact mechanism is
unclear. Isoxaben has been shown to specifically inhibit
the incorporation of glucose into the acid-insoluble fraction
(presumed to be cellulose) of the cell walls of Arabidopsis
thaliana (20) and soybean cell suspension cultures (21).
This herbicide also disrupts cell plate formation in root
tips (22) and tobacco suspension cells (23). Isoxaben affects
a different site of action compared with DCB, as it inhibits
cytokinesis at an earlier stage in which callose is deposited
at the developing cell plate (23). There are no known cases
of resistance to this herbicide. | [Metabolism]
Isoxaben is adsorbed strongly to soil and therefore has
very limited mobility. Volatilization and photodegradation
of isoxaben is negligible when applied to soil. Isoxaben is
mainly degraded by soil organisms and has an average
half-life of 1 to 2 months in the field, providing an average
of 5 to 6 months of weed control at normal rates of
application (19). | [Toxicity evaluation]
Isoxaben is classified as a general use herbicide. Although
it is noncarcinogenic, it is classified as a Class C oncogen
based on increased incidence of benign liver tumors in
one experimental system tested (mice). It is relatively
nontoxic to mammals with an oral LD50 of >10 g/kg in
rats and mice (19).
Isoxaben is nonflammable and noncorrosive. It is stable
under normal conditions, but it is degraded by ultraviolet
light in aqueous solution and decomposes at 220 ?C (19). |
|
|