| Identification | Back Directory | [Name]
Pimaricin | [CAS]
7681-93-8 | [Synonyms]
natacyn
Synogil
cl12,625
delvocid
delvolan
delvopos
mycophyt
myprozine
NATAMYCIN
PIMAFUCIN
PIMARICIN
pimarizin
tennecetin
Myuprozine
d-Natamycin
NataMycin API
Tennecetin (7CI)
antibiotica-5283
Natamycin (200 mg)
Pimaricin(Natamycin)
natamycin preparation
pimaricin preparation
PIMARICIN50%INGLUCOSE
Natamycin50%SodiumChloride
Natamycin(FermentionProcess)
Natamycin50PctMinimum+Lactose
Natamycin (200 mg)J0D1800.917mg/mg(ai)
PIMARICIN, STREPTOMYCES CHATTANOOGENSIS
Pimaricin preparation,Natamycin preparation
pimaricin approx. 2.5% aqueous*suspension gamma-I
Pimaricin, Streptomyces chattanoogensis (1.07360)
pimafucin (pimaricin in 2.5% sterile water suspen
PIMARICIN APPROX. 2.5% AQUEOUS*SUSPENSIO N GAMMA-IRR
Pimafucin (Pimaricin in a 2.5% sterile water suspension)
PIMARICIN, STERILE, AQUEOUS SUSPENSION 2 .5%, PGE W. 20 ML
6,11,28-Trioxatricyclo[22.3.1.05,7]octacosane, pimaricin deriv.
NataMycin, A 5283, Delvolan, Delvocid, Myprozine, Natacyn, Natafucin, PiMafucin, PiMafugin, A5263, CL 12625, E235
6,11,28-Trioxatricyclo22.3.1.05,7octacosa-8,14,16,18,20-pentaene-25-carboxylic acid, 22-(3-amino-3,6-dideoxy-.beta.-D-mannopyranosyl)oxy-1,3,26-trihydroxy-12-methyl-10-oxo-, (1R,3S,5R,7R,8E,12R,14E,16E,18E,20E,22R,24S,25R,26S)-
6,11,28-Trioxatricyclo[22.3.1.05,7]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid, 22-[(3-amino-3,6-dideoxy-b-D-mannopyranosyl)oxy]-1,3,26-trihydroxy-12-methyl-10-oxo-, (1R,3S,5R,7R,8E,12R,14E,16E,18E,20E,22R,24S,25R,26S)-(9CI)
(1R*,3S*,5R*,7R*,8E,12R*,14E,16E,18E,20E,22R*,24S*,25R*,26S*)]-22-[(3-Amino-3,6-dideoxy-β-D-mannopyranosyl)oxy]-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxatricyclo[22.3.1.05,7]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid
[1R-(1R*,3S*,5R*,7R*,8E,12R*,14E,16E,18E,20E,22R*,24S*,25R*,26S*)]-22-[(3-Amino-3,6-dideoxy-b-D-mannopyranosyl)oxy]-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxatricyclo[22.3.1.05,7]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid
6,11,28-Trioxatricyclo[22.3.1.05,7]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid, 22-[(3-amino-3,6-dideoxy-b-D-mannopyranosyl)oxy]-1,3,26-trihydroxy-12-methyl-10-oxo-, [1R-(1R*,3S*,5R*,7R*,8E,12R*,14E,16E,18E,20E,22R*,24S*,25R*,26S*)]- | [EINECS(EC#)]
231-683-5 | [Molecular Formula]
C33H47NO13 | [MDL Number]
MFCD00135085 | [MOL File]
7681-93-8.mol | [Molecular Weight]
665.73 |
| Chemical Properties | Back Directory | [Appearance]
White to Off-White Solid | [Melting point ]
2000C (dec) | [alpha ]
D20 +278° (c = 1 in CH3COOH) | [Boiling point ]
952℃ | [bulk density]
200kg/m3 | [density ]
1.0 g/mL at 20 °C(lit.)
| [refractive index ]
1.5960 (estimate) | [Fp ]
>110°(230°F) | [storage temp. ]
2-8°C
| [solubility ]
Soluble in DMSO | [form ]
aqueous suspension
| [pka]
pKa 4.6(50% aq. MeOEtOH) (Uncertain);8.35 (Uncertain) | [color ]
Cream colored | [Water Solubility ]
0.41g/L(21 ºC) | [Sensitive ]
Light Sensitive | [Merck ]
13,6453 | [BRN ]
1614878 | [Stability:]
Light sensitive | [InChIKey]
NCXMLFZGDNKEPB-FFPOYIOWSA-N | [LogP]
0.880 (est) | [Uses]
Pimaricin is a preservative for use as a coating on the surface of italian cheeses to prevent the growth of mold or yeast. It is tasteless, odorless, colorless, and does not penetrate the cheese. It is very active against virtually all molds and yeasts, but does not affect bacteria, thus not affecting the ripening and flavor improvement process of cheese. It can be applied as a dip, spray, or by other methods such as incorporation into the cheese coatings. It is used at levels ranging from 300 to 2,000 ppm. | [EPA Substance Registry System]
6,11,28-Trioxatricyclo[22.3.1.05,7]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid, 22-[(3-amino-3,6-dideoxy-.beta.-D-mannopyranosyl)oxy]-1,3,26-trihydroxy-12-methyl-10-oxo-, (1R,3S,5R,7R,8E,12R,14E,16E,18E,20E,22R,24S,25R,26S)- (7681-93-8) |
| Hazard Information | Back Directory | [Chemical Properties]
White to Off-White Solid | [Usage]
analgesic, antimigraine | [Usage]
Pimaricin is a macrocyclic tetraene originally isolated from Streptomyces natalensis in 1957. Pimaricin exhibits broad spectrum antifungal activity against yeast and filamentous fungi by binding specifically to ergosterol to block fungal growth. Unlike the related polyenes, nystatin and filipin, pimaricin does not change the permeability of the plasma membrane. Pimaricin is used in the food industry for surface treatment of cheeses as a mould inhibitor. | [Usage]
Polyene antifungal antibiotic | [Originator]
Pimafucine,Beytout,France,1964 | [Manufacturing Process]
The Fermentation Process: The process by which this antifungal substance is
produced is an aerobic fermentation of an aqueous nutrient medium The Fermentation Process: The process by which this antifungal substance is
produced is an aerobic fermentation of an aqueous nutrient medium.
In more detail the nutrient medium used may contain sources of carbon such
as starch, hydrolyzed starch, sugars such as lactose, maltose, dextrose,
sucrose, or sugar sources such as molasses; alcohols, such as glycerol and
mannitol; organic acids, such as citric acid and acetic acid; and various
natural products which may contain other nutrient materials in addition to
carbonaceous substances.
Nitrogen sources include proteins, such as casein, zein, lactalbumin; protein
hydrolyzates such proteoses, peptones, peptides, and commercially available
materials, such as N-Z Amine which is understood to be a casein hydrolyzate;
also corn steep liquor, soybean meal, gluten, cottonseed meal, fish meal,
meat extracts, stick liquor, liver cake, yeast extracts and distillers' solubles;
amino acids, urea, ammonium and nitrate salts. Such inorganic elements as
sodium, potassium, calcium and magnesium; and chlorides, sulfates,
phosphates and combinations of these anions and cations in the form of
mineral salts may be advantageously used in the fermentation.
The so-called trace elements, such as boron, cobalt, iron, copper, zinc,
manganese, chromium, molybdenum and still others may also be used to
advantage. Generally, these trace elements occur in sufficient quantities in the
carbonaceous and nitrogenous constituents of the medium, particularly if
derived from natural sources, or in the tap water, and the addition of further
quantities of these trace elements may consequently be unnecessary.
The fermentation liquor is aerated in the customary manner by forcing sterile
air through the fermenting mixture usually at the rate of about 1 volume of
air per volume of fermentation medium per minute. To minimize
contamination with foreign microorganisms, the fermentation vessels should
be closed and a pressure of 2 to 15 pounds above atmospheric pressure
maintained in the vessel. In addition to the agitation provided by aeration,
mechanical agitation is generally desirable. Antifoaming agents, such as 1%
octadecanol in lard oil, may be added from time to time as required to
prevent excessive foaming. Fermentation is conducted at a temperature
preferably on the order of 26°C to 30°C but may be as low as 17°C or as high
as 42°C.
The time required for maximum production of the antifungal substance will
vary considerably depending upon other conditions of the fermentation.
Generally, about 48 hours is required before appreciable quantities of the
antifungal substance are detected in the medium. The production of the
antifungal substance increases with time, and the fermentation may run as
long as 120 hours. The hydrogen ion conditions normally vary from about pH
6 to pH 8.0, although deviations from these values are permissible, according
to British Patent 846,933. The reader is referred to the patents cited for detals
of pimaricin purification. | [Brand name]
Natacyn (Alcon). | [Therapeutic Function]
Antibacterial (ophthalmic) | [Biological Functions]
Natamycin, also known as pimaracin, belongs to the polyene family of antibiotics; (a group of antifungal agents which target and bind to eukaryotic sterols and specifically ergosterol), and it is a secondary metabolite of Streptomyces natalensis . Very low levels (10–20 ppm) are needed to inhibit almost all yeasts and molds, while no amount of natamycin is sufficient to inhibit most bacteria, as they lack the sterol targeted by natamycin (some gram-positive types may be susceptible). Thus, natamycin may be used to retard the growth of fungi in meat products to which fermentative cultures are added, and is typically applied as a surface treatment (i.e., dip or spray). Resistant organisms are not typically encountered even though natamycin has been used as a food preservative for more than three decades. Unlike most bacteriocins, natamycin is toxic to eukaryotes. Acceptable daily intake of natamycin for humans is 0–0.3 mg/kg of body weight. | [Antimicrobial activity]
The spectrum of Natamycin's activity is somewhat narrower than that of amphotericin and nystatin,
but at the same time, it is less toxic. It exhibits especially pronounced activity against a few
strains of Fusarium and Cefalosporium. Natamycin is a drug for treating superficial fun�gal infections, and it is used only for ophthalmologic purposes. Synonyms of this drug are
pimafucin, pimaricin, tennecetin, and others. | [General Description]
Natamycin (pimaricin; Natacyn) is a polyene antibiotic obtainedfrom cultures of Streptomyces natalensis.The natamycin structure consists of a 26-membered lactonering containing a tetraene chromophore, an α,β-unsaturatedlactone carbonyl group, three hydroxyl groups, a carboxyl group, a trans epoxide, and a glycosidically joined mycosamine.Like the other polyene antibiotics, natamycin isamphoteric.The smaller polyenes are fungistatic and fungicidal within thesame concentration range.Natamycin possesses in vitro activity against severalyeasts and filamentous fungi, including Candida,Aspergillus, Cephalosporium, Penicillium, and Fusariumspp. The drug is supplied as a 5% ophthalmic suspension intendedfor the treatment of fungal conjunctivitis, blepharitis,and keratitis. | [Pharmacokinetics]
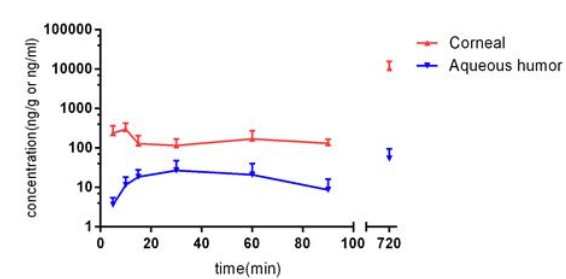
The LC-MS-MS methods allowed Natamycin (Nat) quantification as low as 2.0ng/ml (R>0.99), respectively, for up to 90mins after a single administration of Nat to the eye.
After a single topical instillation, Nat reached the highest concentration in cornea at 10mins (Cmax:299.3ng/g). Nat was detected at all selected time points in the aqueous humor, and the highest was 27.03ng/ml at 30mins. The repeated dosing group, the concentrations in cornea and aqueous humor for Nat were 10569ng/g and 54.4ng/ml, respectively.
 | [Side effects]
Side effects that you should report to doctor as soon as possible:
allergic reactions like skin rash, itching or hives, swelling of the face, lips or tongue
changes in vision
eye pain
severe burning, stinging, or swelling of the eyelids
Side effects that usually do not require medical attention:
blurred vision for a few moments after application
temporary redness or stinging for a few moments after application
temporary watering of eyes | [Synthesis]
Natamycin, a mixture of stereoisomeric 22-[(3-amino-3,6-dideoxy-β-D�mannopyranosyl)oxy]-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxatricy�clo[22.3.1.0.5,7]-octacosa-8,14,16,18,20,penten-25-carboxylic acid (35.1.3), like amphotericin
and nystatin, is a polyene antibiotic that is isolated from the products of the vital activity of
the actinomycete Streptomyces natalensis. | [Veterinary Drugs and Treatments]
Natamycin is a semisynthetic polyene antibiotic. Natamycin is
poorly water-soluble and will not penetrate the intact corneal epithelium.
Natamycin is the only antifungal agent approved for use
on the eye and the only commercially available eye drug for treatment
of fungal keratitis. | [target]
NADPH-oxidase | Antifection |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
22 | [Safety Statements ]
24/25-36 | [WGK Germany ]
2
| [RTECS ]
TK3325000 | [F ]
8-10 | [HS Code ]
29419090 | [Safety Profile]
Poison by intravenous,
intramuscular, subcutaneous, and
intraperitoneal routes. Moderately toxic by
ingestion. When heated to decomposition it emits toxic fumes of NOx. Used as an
antibacterial agent. | [Toxicity]
LD50 orally in male, female rats (g/kg): 2.73, 4.67 (Levinskas) |
| Questions And Answer | Back Directory | [Description]
Pimaricin (also known as natamycin, INN) belongs to a naturally occurring antifungal agent produced through the fermentation of the bacterium Streptomyces natalensis. It is a kind of macrolide polyene antifungal used for the treatment of fungal keratitis, which is a kind of eye infection. In medical field, it can be used for the treatment of various kind of fungal infections caused by Candida, Aspergillus, Cephalosporium, Fusarium, and Penicillium. In food industry, it can be used as a natural preservative to prevent fungal outgrowth. Its mechanism of action is through binding to the ergosterol in the plasma membrane of fungi, inhibiting the process of ergosterol-dependent fusion of vacuoles and membrane fusion, further inhibiting the fungal growth. It also inhibit the transport of amino acid and glucose through inhibiting membrane transport proteins.
| [References]
https://en.wikipedia.org/wiki/Natamycin
https://www.drugbank.ca/drugs/DB00826
|
|
|