| Identification | Back Directory | [Name]
AMBENONIUM | [CAS]
7648-98-8 | [Synonyms]
Win-8077
AMBENONIUM
[Oxalylbis(iminoethylene)]bis[(2-chlorobenzyl)diethylaminium]
N,N'-[(1,2-Dioxo-1,2-ethanediyl)bis(imino-2,1-ethanediyl)]bis[2-chloro-N,N-diethylbenzenemethanaminium]
(2-chlorophenyl)methyl-[2-[[2-[(2-chlorophenyl)methyl-diethyl-ammonio]ethylcarbamoylformyl]amino]ethyl]-diethyl-azanium
(2-chlorophenyl)methyl-[2-[[2-[2-[(2-chlorophenyl)methyl-diethylazaniumyl]ethylamino]-2-oxoacetyl]amino]ethyl]-diethylazanium | [Molecular Formula]
C28H42Cl2N4O2+2 | [MOL File]
7648-98-8.mol | [Molecular Weight]
537.57 |
| Hazard Information | Back Directory | [Definition]
ChEBI: A symmetrical oxalamide-based bis-quaternary ammonium ion having ethyl and 2-chlorobenzyl groups attached to the nitrogens. | [Brand name]
Mytelase (Sanofi Aventis). | [Pharmacology]
The pharmacological properties of ambenonium are similar to neostigmine and pyri�dostigmine, and it works by reversible inactivation of cholinesterase. | [Synthesis]
Ambenonium, [oxalyl-bis-(iminoethylen)]-bis-[ortho-(chlorobenzyl)
diethylammonium] chloride (13.2.15), is made by reacting diethyloxalate with two moles of
N,N-diethylethylendiamine, forming oxalyl-bis-(iminoethylen)-bis-N,N-diethylamine
(13.2.14), which is alkylated by two moles of 2-chlorobenzylchloride, giving ambenonium
(13.2.15) [46¨C48].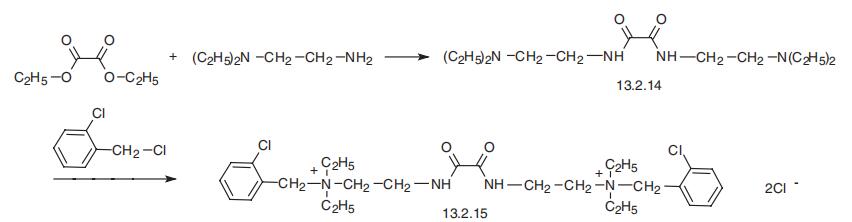
|
|
|