| Identification | Back Directory | [Name]
Ethyl trans-2-(4-Aminocyclohexyl)acetate Hydrochloride | [CAS]
76308-26-4 | [Synonyms]
Cariprazine Impurity 7
Ethyl 2-(trans-4-aminocyclohexyl)
Ethyl trans-2-(4-aminocyclohexyl)acetate, HCl
ETHYL 2-(4-AMINOCYCLOHEXYL)ACETATE;HYDROCHLORIDE
ethyl (trans-4-aminocyclohexyl)acetate hydrochloride
Ethyl 2-(trans-4-aMinocyclohexyl)acetate hydrochloride
Ethyl trans-2-(4-Aminocyclohexyl)acetate Hydrochloride
ethyl 2-[(1r,4r)-4-aminocyclohexyl]acetate hydrochloride
Ethyl (trans-4-aminocyclohexyl)acetate hydrochloride (1:1)
trans-4-Aminocyclohexaneacetic acid ethyl ester hydrochloride
trans-2-(4-Aminocyclohexyl)acetic acid ethyl ester hydrochloride
Cyclohexaneacetic acid, 4-aMino-,ethyl ester (hydrochloride)(1:1), trans- | [Molecular Formula]
C10H20ClNO2 | [MDL Number]
MFCD16660366 | [MOL File]
76308-26-4.mol | [Molecular Weight]
222 |
| Chemical Properties | Back Directory | [Melting point ]
177 °C | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
Methanol (Slightly) | [form ]
Solid | [color ]
White to Off-White | [InChI]
InChI=1/C10H19NO2.ClH/c1-2-13-10(12)7-8-3-5-9(11)6-4-8;/h8-9H,2-7,11H2,1H3;1H/t8-,9-; | [InChIKey]
KBMWRCPPPDEVSJ-JUAUBFSONA-N | [SMILES]
C([C@@H]1CC[C@@H](N)CC1)C(=O)OCC.Cl |&1:1,4,r| |
| Hazard Information | Back Directory | [Uses]
Ethyl trans-2-(4-Aminocyclohexyl)acetate Hydrochloride can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes. | [Synthesis]
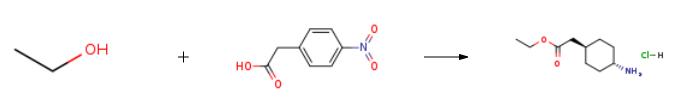
Preparation of trans 4-amino-cyclohexyl-acetic acid ethyl ester HClA 2500 1 enamelled autoclave is charged with 1000 kg of deionizated water and 210 kg (1.16 kM) of 4-nitrophenyl-acetic acid at room temperature under nitrogen atmosphere. After inertisation by nitrogen to the mixture obtained a suspension of 21 kg of 10% Pd/C in 20 kg of deionized water is added and the catalyst measuring gauge is rinsed by additional 20 kg of deionizated water. After rinsing the reaction vessel by hydrogen gas the hydrogenation is carried out at a temperature between 44-46??C and under up to 0,6 bar overpressure until the hydrogen uptake is slowed. After reducing the nitro group the temperature is brought to 55- 58??C and the hydrogenation continued maintaining hydrogen pressure on max. 4.0 bar overpressures. After completion of the hydrogen uptake, the mixture is cooled to a temperature between 25-30??C, purged with nitrogen and the catalyst is filtered on a Spakler filter under pressurized nitrogen. The reaction vessel, the filter and the lines are washed additional 200 g of deionized water. The filtrates are combined and in a 2500 1 enamelled doubler 1200 kg of distillate is distilled at up to 80??C inner temperature in vacuum. The residue obtained is cooled to a temperature below 30??C and 430 kg of ethyl alcohol is added, then 500 1 of distillate is collected at up to 80??C under vacuum. After completion of the distillation the mixture is cooled to a temperature between 25-30??C, (water content is max. 10 w%, in terms of absolute value is about 32 kg), and 550 kg of ethyl alcohol, then 170 kg of 30% hydrochloric ethyl alcohol are added and the reaction mixture is heated to reflux for approx. 2 hours. When the esterification is complete 800 1 of solvent is distilled off at up to 80??C, under vacuum. Additional 800 1 of ethyl alcohol is added and further 750-800 1 of solvent is distilled off at up to 80??C, under vacuum. To the residue obtained 700 kg of acetonitrile is added and 140 1 of distillate is collected at up to 80??C under vacuum. The vacuum is stopped by introducing nitrogen and the solution is cooled to a temperature between 0-(-)5??C. The crystals obtained is centrifuged, washed with 100 kg of acetonitrile in two portions during which the temperature is kept at 0-(-)5??C. The solid obtained is dried to a constant weight at up to 6O0C. In this manner 90 kg of title product is obtained. Yield: 40 %. Melting point: 173-1760C. |
|
|