| Identification | Back Directory | [Name]
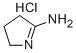
2-AMINO-1-PYRROLINE HYDROCHLORIDE | [CAS]
7544-75-4 | [Synonyms]
Tipiracil-004-HCl
2-Aminopyrrolidine
Tipiracil Impurity 13
2-AMinopyrrolidine HCl
2-Amino-1-pyrroline HCl
2-iminopyrrolidine-hydrochloride
3,4-DIHYDRO-2H-PYRROL-5-AMINE HCL
2-AMINO-1-PYRROLINE HYDROCHLORIDE
2-Aminopyrrolidine hydrochloride
2-Amino-1-pyrrolidine hydrochloride
2-amino-4,5-dihydro-3H-pyrrole HCl salt
4,5-dihydro-3H-pyrrol-2-amine hydrochloride
3,4-Dihydro-2H-pyrrol-5-aMine hydrochloride
5-Amino-3,4-dihydro-2H-pyrrole Hydrochloride
4,5-Dihydro-3H-pyrrol-2-ylamine hydrochloride
2-amino-4,5-dihydro-3H-pyrrole (hydrochloride)
3,4-dihydro-2H-pyrrol-5-amine hydrochloride (1:1)
2H-Pyrrol-5-amine, 3,4-dihydro-, monohydrochloride
2H-Pyrrol-5-amine, 3,4-dihydro-, hydrochloride (1:1) | [Molecular Formula]
C4H9ClN2 | [MDL Number]
MFCD00790772 | [MOL File]
7544-75-4.mol | [Molecular Weight]
120.581 |
| Hazard Information | Back Directory | [Synthesis]
In a 250 mL bottomed flask, ammonia was bubbled into ethanol (80 mL) that was cooled to -78° C. for 45 min. The saturated solution that was obtained was transferred to a Parr autoclave, which contained 4-chlorobutanenitrile (60 g, 580 mmol). The mixture was stirred and heated to 120° C for 16 hr. The reaction mixture was cooled and transferred to a round-bottomed flask. The solvent was removed under reduced pressure to afford a yellow solid, which was washed with diethyl ether and dried under a high vacuum to afford an off-white solid 2-Amino-1-pyrroline Hydrochloride, 19.5 g, 232 mmol, 40percent yield. 1H NMR (DMSO-d6) δ 9.22 (br s, 1H), 8.93 (br s, 1H), 7.60 (br s, 1H), 3.52 (d, J=7.2 Hz, 2H), 2.76 (d. J=8.0 Hz, 2H), 2.08-1.96 (m, 2H). |
|
|