| Identification | Back Directory | [Name]
DEOXYGALACTONOJIRIMYCIN, HYDROCHLORIDE | [CAS]
75172-81-5 | [Synonyms]
DGJ
Amigal
Migalastat HCl
Unii-cly7m0xd20
GALACTOSTATIN HCL
DGJ, HYDROCHLORIDE
Migalastat hydrochloride
Galactostatin hydrochloride
DEOXYGALACTONOJIRIMYCIN HCL
1-DEOXYGALACTONOJIRIMYCIN HCL
1,5-dideoxy-1,5-imino-d-galactitol
1-Deoxygalactostatin Hydrochloride
DEOXYGALACTONOJIRIMYCIN, HYDROCHLORIDE
1-Deoxygalactonojirimycin Hydrochloride
1,5-DIDEOXY-1,5-IMINO-D-GALACTITOL, HYDROCHLORIDE
(2R,3S,4R,5S)-2-HydroxyMethyl-3,4,5-piperidinetriol Hydrochloride
Galactostatin hydrochloride, Deoxygalactonojirimycin hydrochloride
(2R,3S,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-triol hydrochloride
3,4,5-Piperidinetriol, 2-(hydroxymethyl)-, hydrochloride, (2R,3S,4R,5S)- | [Molecular Formula]
C6H14ClNO4 | [MDL Number]
MFCD00269962 | [MOL File]
75172-81-5.mol | [Molecular Weight]
199.63 |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
260 | [storage temp. ]
2-8°C
| [solubility ]
Methanol (Slightly), Water (Slightly) | [form ]
White crystalline solid | [color ]
White to Brown | [biological source]
synthetic (organic) | [Water Solubility ]
water: soluble 1mg/mL |
| Hazard Information | Back Directory | [Chemical Properties]
White Crystalline Solid | [Uses]
inhibitor of b-glucosidase | [Uses]
Proven to be an extremely potent and selective a-D-galactosidase inhibitor. | [Description]
Migalastat,
which is marketed by Amicus Therapeutics, received approval
in the EU for the treatment of Fabry disease in adults and
adolescents aged 16 or older. Fabry disease is caused by
mutations of the enzyme α-galactosidase A (α-GAL A) that
cause protein misfolding and prevents efficient metabolism of
the glycosphingolipid globotriaosylceramide (GL3). Accumulation
of GL3 in lysosomes, blood vessels, and various tissues
ultimately leads to significant heart, kidney, and dermatological
problems. Migalastat functions as a molecular chaperone to α-
GAL A, engaging the enzyme and enabling it to adopt the
proper conformation allowing for efficient breakdown of
GL3. Because the standard of care prior to 2016 for treating
Fabry disease was enzyme replacement therapy (ERT),
migalastat’s approval in the EU represents an important
advance for patients suffering from this disorder. | [Brand name]
Treatment of Fabry
disease. | [Biochem/physiol Actions]
Deoxygalactonojirimycin hydrochloride is an inhibitor of α-galactosidase A. Deoxygalactonojirimycin exhibits therapeutic effects against Fabry disease. | [Synthesis]
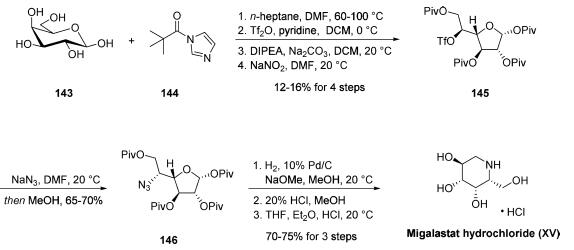
Several unique synthetic approaches to migalastat, which is
also known as D-1-deoxygalactonojirimycin (DGJ), have been
reported in the literature. Although the most likely
commercial-scale preparation of this drug proceeds through a
microbial fermentation process disclosed in a 2015 patent, a
kilogram-scale synthesis of the drug outlined has
been described in a 2008 patent application filed by Amicus.
This route closely resembles a procedure disclosed in 1999 by
Uriel and Santoyo-Gonzalez that presented handling and
safety concerns. Commercial D-galactose (143) was treated
with five equivalents of pivaloyl imidazole (144), followed by
triflation, treatment with Hunig?ˉs base, and exposure to sodium
nitrite to furnish the tetrapivaloyl altofuranose triflate 145 after
recrystallization from heptane. Next, stereospecific azide
displacement of the triflate successfully delivered azidofuranose 146 in 65-70% yield. This reaction generated over 3 kg of the
desired alkyl azide after recrystallization from ethanol and
water. Lastly, palladium-catalyzed hydrogenolysis in the
presence of sodium methoxide, a methanolic acidification
step, and then a subsequent acidification step using HCl in
THF furnished migalastat hydrochloride (XV) in 70-75% yield
over the three-step sequence from 146.
 | [target]
α-galactosidase | [IC 50]
40 nm | [References]
[1] asano n, ishii s, kizu h, et al. in vitro inhibition and intracellular enhancement of lysosomal α‐galactosidase a activity in fabry lymphoblasts by 1‐deoxygalactonojirimycin and its derivatives[j]. febs journal, 2000, 267(13): 4179-4186.
[2] ishii s, chang h, yoshioka h, et al. preclinical efficacy and safety of 1-deoxygalactonojirimycin in mice for fabry disease[j]. journal of pharmacology and experimental therapeutics, 2009, 328(3): 723-731. |
|
|