| Identification | Back Directory | [Name]
(5AS, 10BR)-(-)-5A,10B-DIHYDRO-2-(PENTAFLUOROPHENYL)-4H,6H-INDENO[2,1-B][1,2,4]TRIZOLO[4,3-D][1,4]OXAZINIUM TETRAFLUOROBORATE | [CAS]
740816-14-2 | [Synonyms]
5a(S),10b(R)-5a,10b-Dihydro-2-(pentafluorophenyl)-4H,6H-indeno[2
2-PENTAFLUOROPHENYL-6.10B-DIHYDRO-4H,5AH-5-OXO-3,10C-DIAZA-2-AZONIACYCLOPENTA[C]FLUORENE TETRAFLUOROBORATE
5A(S),10B(R)-5A,10B-DIHYDRO-2-(PENTAFLUOROPHENYL)-4H, 6H-INDENO(2,1-B)(1,2,4)TRIAZOLO(4,3-D)(1,4)OXAZINIUM TETRAFLUOROB
5A(S),10B(R)-5A,10B-DIHYDRO-2-(PENTAFLUOROPHENYL)-4H,6H-INDENO[2,1-B][1,2,4]TRIAZOLO[4,3-D][1,4]OXAZINIUM TETRAFLUOROBORATE
(5AS, 10BR)-(-)-5A,10B-DIHYDRO-2-(PENTAFLUOROPHENYL)-4H,6H-INDENO[2,1-B][1,2,4]TRIZOLO[4,3-D][1,4]OXAZINIUM TETRAFLUOROBORATE
5a(S),10b(R)-5a,10b-Dihydro-2-(pentafluorophenyl)-4H,6H-indeno[2,1-b][1,2,4]triazolo[4,3-d][1,4]oxazinium tetrafluoroborate 97%
(5aS,10bR)-(-)-5a,10b-Dihydro-2-(pentafluorophenyl)-4H,6H-indeno[2,1-b][1,2,4]trizolo[4,3-d][1,4]oxaziniumtetrafluoroborate,min.98%
(5aS,10bR)-5a,10b-dihydro-2-(2,3,4,5,6-pentafluorophenyl)-4H,6H-Indeno[2,1-b][1,2,4]triazolo[4,3-d][1,4]oxazinium tetrafluoroborate
(5aS, 10bR)-(-)-5a,10b-Dihydro-2-(pentafluorophenyl)-4H,6H-indeno[2,1-b][1,2,4]trizolo[4,3-d][1,4]oxazinium tetrafluoroborate, min. 98%
(5aS, 10bR)-(-)-5a,10b-Dihydro-2-(pentafluorophenyl)-4H,6H-indeno[2,1-b][1,2,4]triazolo[4,3-d][1,4]oxazinium tetrafluoroborate, min. 98%
(5aS,10bR)-5a,10b-Dihydro-2-(2,3,4,5,6-pentafluorophenyl)-4H,6H-indeno[2,1-b][1,2,4]triazolo[4,3-d][1,4]oxazinium Tetrafluoroborate,99%e.e.
(5aS,10bR)-(-)-5a,10b-Dihydro-2-(pentafluorophenyl)-4H,6H-indeno[2,1-b]
[1,2,4]trizolo[4,3-d][1,4]oxazinium tetrafluoroborate, min. 98%
(5aR,10bS)-(+)-5a,10b-Dihydro-2-(pentafluorophenyl)-4H,6H-indeno[2,1-b]
[1,2,4]triazolo[4,3-d][1,4]oxazinium tetrafluoroborate, min. 98% | [Molecular Formula]
C18H11BF9N3O | [MDL Number]
MFCD08459334 | [MOL File]
740816-14-2.mol | [Molecular Weight]
467.1 |
| Questions And Answer | Back Directory | [Reaction]
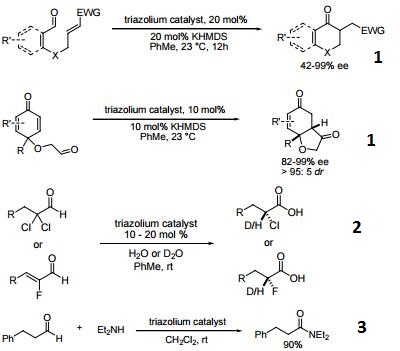
- Reagent used in the highly enantioand diastereoselective, catalytic intramolecular Stetter reaction.
- Direct synthesis of α-protio and α-deuterio α-chloro and α-fluoro carboxylic acids via assymetric hydration.
- Chemoselective conversion of α-unbranched aldehydes to amides.
|
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
| Company Name: |
Alfa Chemistry
|
| Tel: |
1-516-6625404 |
| Website: |
https://www.alfa-chemistry.com |
| Company Name: |
LaaJoo
|
| Tel: |
021-60702684 18516024827 |
| Website: |
http://m.is0513.com/ShowSupplierProductsList20079/0.htm |
|